Nuclear chain reaction
Nuclear chain reactions are reactions where nuclear energy is obtained, generally through nuclear fission. These chain reactions are what provide nuclear power plants with the energy that is then turned into electricity for use by people. In these reactions, neutrons generated by the fission process continue on to initiate fission in other atoms. These reactions generally occur with heavier isotopes such as uranium-235 where there is a continuous release and absorption of neutrons. Figure 1 below is a visual representation of what a nuclear fission chain reaction looks like. If at least one neutron from each fission strikes another U-235 nucleus and initiates fission, the chain reaction is sustained and it is said to be critical.
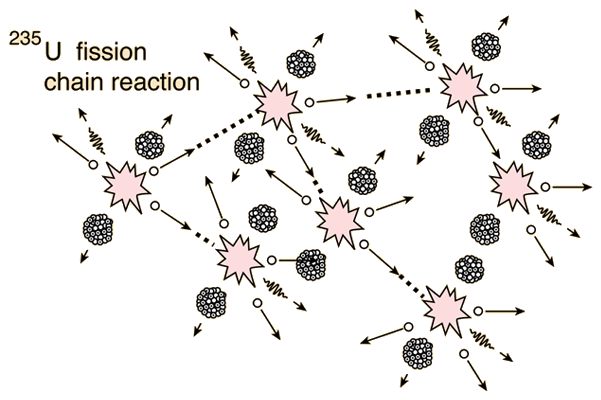
The mass of uranium-235 that is required to produce a reaction that is self-sustaining is said to be the "critical mass". This critical chain reaction can be accomplished at relatively low uranium-235 concentrations if the neutrons are moderated to lower their speed. A moderator is necessary because the probability for fission is greater with slow neutrons.[1] The general equation for a nuclear chain reaction of uranium-235 is:[2]
If for every one neutron inputted into the equation releases two or three more neutrons, then the number of fission events increases dramatically each generation. However, in reality not all of the released neutrons actually cause more fissions. Only 1.1 neutron per reaction actually goes on to cause more fissions and continue the chain, however the number of fission events still grows quickly. The process of a nuclear chain reaction releases large amounts of energy, but this energy can be utilized in different ways. On average, there is about 200 MeV of energy released during fission.[2] To put this into context, burning coal provides only a couple eV, while 200 MeV is equal to 200 million electron volts.[3] The difference in these energies is enormous. In nuclear reactors, the reaction is moderated and progresses at a slow pace to release its energy over a period of time so it can be harnessed and used for peaceful purposes. An atomic bomb utilizes this fission chain reaction as well, however it is designed to release its energy all at once—which is much more damaging. In either case, the release of the energy is controlled, but the time period taken to release the energy differs.[2]
For more information on nuclear chain reactions see HyperPhysics here.
References
- ↑ 1.0 1.1 HyperPhysics. (May 27, 2015). Uranium-235 Chain Reaction [Online]. Available: http://hyperphysics.phy-astr.gsu.edu/hbase/nucene/u235chn.html
- ↑ 2.0 2.1 2.2 Atomic Archive. (June 16, 2015). Nuclear Chain Reactions [Online]. Available: http://www.atomicarchive.com/Fission/Fission2.shtml
- ↑ UC Davis' Chem Wiki. (July 9, 2015). Fission and Fusion [Online]. Available: http://chemwiki.ucdavis.edu/Physical_Chemistry/Nuclear_Chemistry/Fission_and_Fusion

