Solute
A solute is simply a substance that dissolves in some other substance - known as a solvent - to form a solution. Although a solute is generally thought of as being solid, a solute can be either solid, liquid, or gas.[2] Based on the chemical properties of both the solvent and the solute, the amount of solute that will dissolve in a solvent will vary. The solubility of a solute depends on the temperature, volume and mass ratio, and other chemical properties of the substances.[3]
To learn more about solutes and solutions please see UC Davis' Chem wiki.
CO2 as a Solute
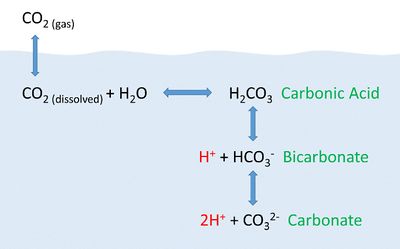
A solute does not have to be a solid, it can be a gas like carbon dioxide (CO2). Carbon dioxide in the atmosphere is soluble in water (the solvent), which means it can become dissolved CO2 in the oceans. As seen in figure 2, the dissolved carbon dioxide then undergoes chemical reactions with water to produce carbonic acid (H2CO3), bicarbonate (HCO3–), carbonate (CO32-) and hydrogen ions (H+). Most of the CO2 dissolving or produced in the ocean is quickly converted to bicarbonate and hydrogen ions.[4]
The more CO2 is dissolved in the oceans, the more hydrogen ions are produced which consequently lowers the pH of the solution (the ocean in this case). As anthropogenic sources of atmospheric CO2 have increased, the oceans have been absorbing an increasing amount of carbon dioxide. As a result, there has been a decline in ocean pH and an increase in acidity.[4]
Please see the page about ocean acidification for more information.
Further Reading
- Solvent
- Solid, Liquid
- Chemical reaction
- pH
- Or explore a random page
References
- ↑ "Difference between Solute and Solvent," LaboratoryInfo, 2021. [Online]. Available: https://laboratoryinfo.com/solute-vs-solvent/. [Accessed: 12-May-2021]
- ↑ "Solute and Solvent," Chemistry LibreTexts, 2021. [Online]. Available: https://chem.libretexts.org/@go/page/53840. [Accessed 12-May-2021].
- ↑ Solubility. BCcampus Open Publishing. [Online]. Available: https://opentextbc.ca/chemistry/chapter/11-3-solubility/. [Accessed: 13-May-2021]
- ↑ 4.0 4.1 4.2 "Dissolved Gases: Carbon Dioxide, pH, and Ocean Acidification," Roger Williams University Pressbooks. [Online]. Available: https://rwu.pressbooks.pub/webboceanography/chapter/5-5-dissolved-gases-carbon-dioxide-ph-and-ocean-acidification/. [Accessed: 13-May-2021]