Acid

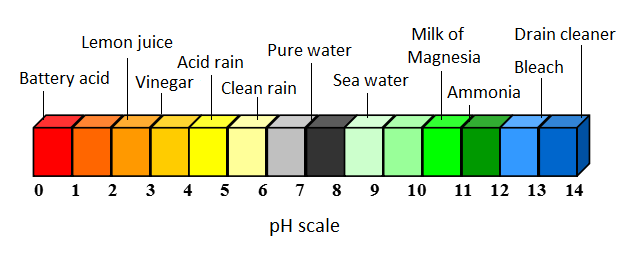
An acid is any substance with a pH less than 7. This means that the ion concentration of hydrogen (H+) is larger than the concentration of hydroxide (OH-). A lower pH means a higher acidity, and thus a higher concentration of H+ ions in the solution. These types of substances exhibit distinct properties: they taste sour and produce a piercing pain in contact with a wound (a lemon, for instance).[2]
When mixed with a base, the pH of the new solution will balance out towards the neutral pH of 7, depending on the strength of the acid and base. This is known as neutralization.[2]
Acid rain is an environmental problem caused by pollution from energy use. Acid rain, acid snow and even acid fog, (all collectively referred to as just acid rain) forms when water interacts with pollutants in the atmosphere. It has a lower pH than normal rain, and can be more than 25 times more acidic than clean rain. Visit the acid rain page for more information.
Uses
Acids have many uses. They can be used to remove rust from metals, as an electrolyte in a wet-cell battery (such as a car battery), in the chemical industry to produce many desired products, as additives to drinks or food to alter taste and act as a preservative (in soda for example), and occur naturally in fruits and vegetables.
Acids are also important in the human body. They act to break down food, are used for protein synthesis, tissue repair, and pH balancing. Ascorbic acid (Vitamin C) is essential for the human body, as well as many other acids. The "A" in DNA and RNA stands for acid, and these are crucial molecules for all life.
To learn more about acids and bases please see UC Davis's chem wiki.
References
- ↑ Wikimedia Commons [Online], Available: https://commons.wikimedia.org/wiki/File:Lemon.jpg
- ↑ 2.0 2.1 UC Davis Chem Wiki. (July 8, 2015). Acid/Base Basics [Online], Available: http://chemwiki.ucdavis.edu/Physical_Chemistry/Acids_and_Bases/Acid#Neutralization
- ↑ Adapted from Energy: Its use and the Environment -- R. A. Hinrichs and M. Kleinbach, "Acid Rain," in Energy: Its Use and the Environment, 5th ed. Toronto, Ont. Canada: Brooks/Cole, 2006, ch.8, sec.C, pp.252-256

