Mercaptan: Difference between revisions
J.williams (talk | contribs) m (1 revision imported) |
energy>Jmdonev No edit summary |
||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2018-08-03]] | ||
[[File:640px-Methanethiol-3D-balls.png|400px|framed|right|Figure 1. A model of mercaptan. Here the yellow represents sulfur, the black represents carbon, and the white represents hydrogen.<ref>Wikimedia Commons. (June 15, 2015). ''Methanethiol'' [Online]. Available: http://commons.wikimedia.org/wiki/File:Methanethiol-3D-balls.png#/media/File:Methanethiol-3D-balls.png</ref>]] | [[File:640px-Methanethiol-3D-balls.png|400px|framed|right|Figure 1. A model of mercaptan. Here the yellow represents sulfur, the black represents carbon, and the white represents hydrogen.<ref>Wikimedia Commons. (June 15, 2015). ''Methanethiol'' [Online]. Available: http://commons.wikimedia.org/wiki/File:Methanethiol-3D-balls.png#/media/File:Methanethiol-3D-balls.png</ref>]] | ||
<onlyinclude>'''Mercaptan''', also known as '''methanethiol''' is a foul-smelling [[gas]] that is added to [[natural gas]] | <onlyinclude>'''Mercaptan''', also known as '''methanethiol''' is a foul-smelling [[gas]] that is added to [[natural gas]]. Since natural gas is colourless and odourless, mercaptan acts as an [[odorant]] to make it easier to detect.</onlyinclude><ref name="RE2">Columbia Gas. (June 15, 2015). ''What is Mercaptan'' [Online]. Available: https://www.columbiagasohio.com/stay-safe/what-to-do-when-you-smell-gas/what-is-mercaptan-</ref> It is added as a safety measure to ensure that natural gas leaks do not go undetected. It is an organic gas composed of [[carbon]], [[hydrogen]], and [[sulfur]]. Mercaptan is found naturally in living organisms, including the human body where it is a [[pollution vs waste|waste]] product of metabolism. | ||
Mercaptans bond strongly with [[mercury]] compounds, and most | Mercaptans bond strongly with [[mercury]] compounds, and most release strong odours that resemble garlic or rotting cabbage.<ref name="RE1">Maria Mergel. (June 15, 2015). ''Mercaptans'' [Online]. Available: http://www.toxipedia.org/display/toxipedia/Mercaptans</ref> These compounds are detectable by the human nose at [[concentration in parts per|concentration]]s as small as only 10 [[parts per billion]], making them an effective odourant.<ref name="RE2"/> Natural gas distributors began adding these mercaptans to natural gas after a deadly school explosion in 1937 at the New London School in New London, Texas. Currently, most gas odorants are mixtures of mercaptans and sulfides.<ref name="RE1"/> | ||
Although many mercaptans have foul odours, there are exceptions. For example, "grapefruit mercaptan" is responsible for the characteristic scent of grapefruit.<ref name="RE1"/> | Although many mercaptans have foul odours, there are exceptions. For example, "grapefruit mercaptan" is responsible for the characteristic scent of grapefruit.<ref name="RE1"/> | ||
==For Further Reading== | |||
*[[Odorant]] | |||
*[[Natural gas]] | |||
*[[Mercury]] | |||
*[[Gas]] | |||
*[[Concentration in parts per]] | |||
*[[Pollution vs waste]] | |||
*Or explore a [[Special:Random|random page]] | |||
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
[[Category:Uploaded]] | [[Category:Uploaded]] | ||
Revision as of 21:28, 12 August 2018
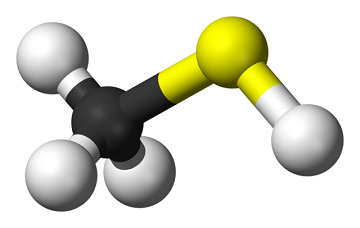
Mercaptan, also known as methanethiol is a foul-smelling gas that is added to natural gas. Since natural gas is colourless and odourless, mercaptan acts as an odorant to make it easier to detect.[2] It is added as a safety measure to ensure that natural gas leaks do not go undetected. It is an organic gas composed of carbon, hydrogen, and sulfur. Mercaptan is found naturally in living organisms, including the human body where it is a waste product of metabolism.
Mercaptans bond strongly with mercury compounds, and most release strong odours that resemble garlic or rotting cabbage.[3] These compounds are detectable by the human nose at concentrations as small as only 10 parts per billion, making them an effective odourant.[2] Natural gas distributors began adding these mercaptans to natural gas after a deadly school explosion in 1937 at the New London School in New London, Texas. Currently, most gas odorants are mixtures of mercaptans and sulfides.[3]
Although many mercaptans have foul odours, there are exceptions. For example, "grapefruit mercaptan" is responsible for the characteristic scent of grapefruit.[3]
For Further Reading
- Odorant
- Natural gas
- Mercury
- Gas
- Concentration in parts per
- Pollution vs waste
- Or explore a random page
References
- ↑ Wikimedia Commons. (June 15, 2015). Methanethiol [Online]. Available: http://commons.wikimedia.org/wiki/File:Methanethiol-3D-balls.png#/media/File:Methanethiol-3D-balls.png
- ↑ 2.0 2.1 Columbia Gas. (June 15, 2015). What is Mercaptan [Online]. Available: https://www.columbiagasohio.com/stay-safe/what-to-do-when-you-smell-gas/what-is-mercaptan-
- ↑ 3.0 3.1 3.2 Maria Mergel. (June 15, 2015). Mercaptans [Online]. Available: http://www.toxipedia.org/display/toxipedia/Mercaptans

