Orbital
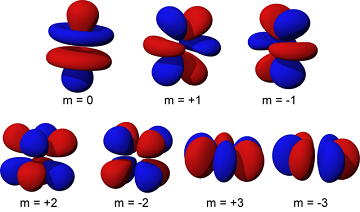
An orbital in an atom refers to the regions within the atom where electrons have the highest probability of being found, since electrons are dispersed through a large volume of the atom and not concentrated in one area like the neutrons and protons. The specifics as to why electrons are found more commonly in certain areas of the atom become complex very quickly, as the probabilities are based in principles of quantum mechanics.[2]
Simple understandings of orbitals describe the areas where electrons can be found as "layers" in a cloud of electrons, spherical areas where electrons are more likely to be found. However, quantum mechanics describes these orbitals quite differently, including values known as quantum numbers to describe the size and shape of these orbitals. The orbital that an electron lies within determines the energy that the electron has, and orbitals that result in two electrons having the same energy while being in a different orbital are referred to as degenerate.[3]
Although orbitals are complex, their structure can explain properties of some matter. For example, by knowing whether or not an orbital is full (each has room for two electrons),half full or empty determines the stability of an element since the orbitals "want" to be full or half full. For example, an element like copper is a stable element.[3] In addition to this, many of the periodic properties of certain elements and the properties that families of elements have is caused by how these orbitals are filled with electrons.[4]
To learn more about orbitals from a chemistry perspective, see UC Davis' Chemwiki.
To learn more about orbitals from a physics perspective, see Hyperphysics.
References
- ↑ Wikimedia Commons. (May 15, 2015). F Orbital [Online]. Available: http://commons.wikimedia.org/wiki/File:F_orbital.png#/media/File:F_orbital.png
- ↑ J.Kotz, J.Townsend, P.Treichel. (May 15, 2015). Chemistry and Chemical Reactivity, 8th ed. Belmont, CA, U.S.A: Brooks/Cole, 2012.
- ↑ 3.0 3.1 UC Davis Chemwiki. (May 15, 2015). Electronic Orbitals [Online]. Available: http://chemwiki.ucdavis.edu/Physical_Chemistry/Quantum_Mechanics/09._The_Hydrogen_Atom/Atomic_Theory/Electrons_in_Atoms/Electronic_Orbitals
- ↑ HyperPhysics. (May 15, 2015). Visualizing Electron Orbitals [Online]. Available: http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/eleorb.html

