Ozone: Difference between revisions
J.williams (talk | contribs) m (1 revision imported) |
m (1 revision imported) |
||
| (One intermediate revision by one other user not shown) | |||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2021-01-31]] | ||
<onlyinclude>'''Ozone''' is a [[molecule]] of [[oxygen]] with the formula O<sub>3</sub>.</onlyinclude> | <onlyinclude>'''Ozone''' is a [[molecule]] made of three of [[oxygen]] [[atom]]s with the formula O<sub>3</sub>.</onlyinclude> Ozone is a reactive oxidant gas produced naturally in trace amounts in Earth’s [[atmosphere]]. However the location of it within the atmosphere is crucial. In the [[troposphere]] (the atmospheric layer closest to Earth's surface), it can be harmful to humans. In the [[stratosphere]] (the atmospheric layer above the troposphere) it is vital in protecting the Earth from harmful [[ultraviolet]] radiation. Ozone in the stratosphere can be referred to as the "[[ozone layer]]".<ref name=atm>Seinfeld, John H, and Pandis, Spyros N. ''Atmospheric Chemistry and Physics.'' John Wiley & Sons, Incorporated, 2016.</ref> Because ozone functions differently in different parts of the atmosphere, its use in conversation can be ambiguous: | ||
*For harmful ozone that is viewed as a [[pollutant]], visit '''[[ground level ozone]]'''. | *For harmful ozone that is viewed as a [[pollutant]], visit '''[[ground level ozone]]'''. | ||
*For ozone that protects the Earth and is important to the success of living organisms, visit '''[[ozone layer]]'''. | *For ozone that protects the Earth and is important to the success of living organisms, visit '''[[ozone layer]]'''. | ||
[[File:good and bad ozone.png|800px|center|thumb|framed|Figure 1: Diagram of ozone in different regions in the atmosphere. Ozone in the upper atmosphere protects from ultraviolet radiation, while ozone in the lower atmosphere acts as a greenhouse gas and can be harmful to human health.<ref>"Ozone Trends," ETH Zurich: Institute for Atmospheric and Climate Science, 2021. [Online]. Available: https://iac.ethz.ch/group/atmospheric-chemistry/research/ozone-trends.html. [Accessed: 18-May-2021]</ref>]] | |||
===Stratospheric Ozone=== | |||
Most of Earth’s atmospheric ozone (about 90%) is found in the stratosphere where it plays a critical role in absorbing [[ultraviolet radiation]] emitted by the [[sun]]. The levels of stratospheric ozone are very important and a thinning ozone layer can cause problems. The thinning of naturally occurring ozone at the poles (commonly known as the "ozone hole") is due to [[photochemical reaction]]s with [[chemical]]s such as chlorofluorocarbons. A reduction in stratospheric ozone leads to increased levels of UV-B radiation at the ground, which can lead to increased risks of skin cancer. Recovering levels of ozone in the upper atmosphere (repairing the hole in the ozone layer) is a good thing, which is the opposite of increasing the levels of ozone in the lower atmosphere.<ref name=atm/> | |||
===Tropospheric Ozone=== | |||
Within the last 70 years, it was realized that [[anthropogenic]] emissions could lead to ozone increases in the troposphere. Tropospheric ozone is considered an [[air pollutant]] as it causes respiratory effects in humans and impairs plant growth. Ozone in the lower atmosphere is mostly produced by photochemical reactions involving [[volatile organic compound]]s and [[nitrous oxide|oxides of nitrogen]]. These compounds have natural sources like vegetation, wildfires, and lightning, and [[anthropogenic]] sources such as [[fossil fuel]] combustion and human-caused [[biomass]] burning. Tropospheric ozone is also an important [[greenhouse gas]] contributing to [[global warming]].<ref name=atm/> For more information about ozone in the lower atmosphere, visit the page on [[ground level ozone]]. | |||
==For Further Reading== | |||
*[[Ground level ozone]] | |||
*[[Secondary pollutant]] | |||
*[[Radiation]] | |||
*[[Ultraviolet]] | |||
*Or explore a [[Special:Random|random page]] | |||
==References== | |||
{{reflist}} | |||
[[Category:Uploaded]] | [[Category:Uploaded]] | ||
Latest revision as of 19:13, 15 October 2021
Ozone is a molecule made of three of oxygen atoms with the formula O3. Ozone is a reactive oxidant gas produced naturally in trace amounts in Earth’s atmosphere. However the location of it within the atmosphere is crucial. In the troposphere (the atmospheric layer closest to Earth's surface), it can be harmful to humans. In the stratosphere (the atmospheric layer above the troposphere) it is vital in protecting the Earth from harmful ultraviolet radiation. Ozone in the stratosphere can be referred to as the "ozone layer".[1] Because ozone functions differently in different parts of the atmosphere, its use in conversation can be ambiguous:
- For harmful ozone that is viewed as a pollutant, visit ground level ozone.
- For ozone that protects the Earth and is important to the success of living organisms, visit ozone layer.
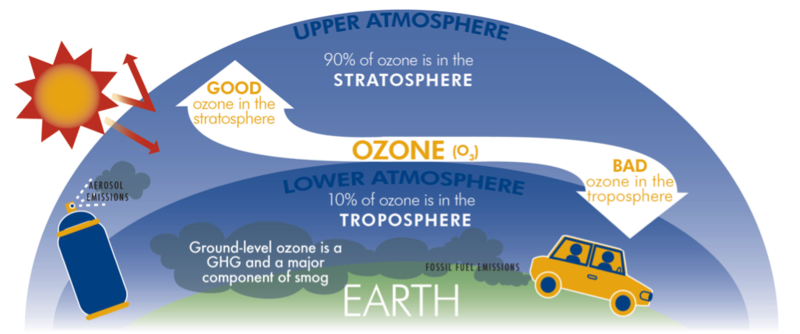
Stratospheric Ozone
Most of Earth’s atmospheric ozone (about 90%) is found in the stratosphere where it plays a critical role in absorbing ultraviolet radiation emitted by the sun. The levels of stratospheric ozone are very important and a thinning ozone layer can cause problems. The thinning of naturally occurring ozone at the poles (commonly known as the "ozone hole") is due to photochemical reactions with chemicals such as chlorofluorocarbons. A reduction in stratospheric ozone leads to increased levels of UV-B radiation at the ground, which can lead to increased risks of skin cancer. Recovering levels of ozone in the upper atmosphere (repairing the hole in the ozone layer) is a good thing, which is the opposite of increasing the levels of ozone in the lower atmosphere.[1]
Tropospheric Ozone
Within the last 70 years, it was realized that anthropogenic emissions could lead to ozone increases in the troposphere. Tropospheric ozone is considered an air pollutant as it causes respiratory effects in humans and impairs plant growth. Ozone in the lower atmosphere is mostly produced by photochemical reactions involving volatile organic compounds and oxides of nitrogen. These compounds have natural sources like vegetation, wildfires, and lightning, and anthropogenic sources such as fossil fuel combustion and human-caused biomass burning. Tropospheric ozone is also an important greenhouse gas contributing to global warming.[1] For more information about ozone in the lower atmosphere, visit the page on ground level ozone.
For Further Reading
- Ground level ozone
- Secondary pollutant
- Radiation
- Ultraviolet
- Or explore a random page
References
- ↑ 1.0 1.1 1.2 Seinfeld, John H, and Pandis, Spyros N. Atmospheric Chemistry and Physics. John Wiley & Sons, Incorporated, 2016.
- ↑ "Ozone Trends," ETH Zurich: Institute for Atmospheric and Climate Science, 2021. [Online]. Available: https://iac.ethz.ch/group/atmospheric-chemistry/research/ozone-trends.html. [Accessed: 18-May-2021]

