Rankine cycle
The Rankine cycle or Rankine Vapor Cycle is the process widely used by power plants such as coal-fired power plants or nuclear reactors. In this mechanism, a fuel is used to produce heat within a boiler, converting water into steam which then expands through a turbine producing useful work. This process was developed in 1859 by Scottish engineer William J.M. Rankine.[1] This is a thermodynamic cycle which converts heat into mechanical energy—which usually gets transformed into electricity by electrical generation.
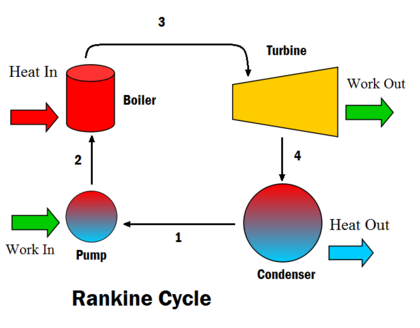
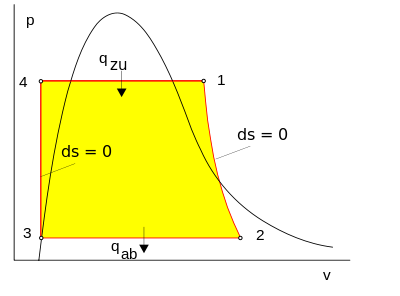
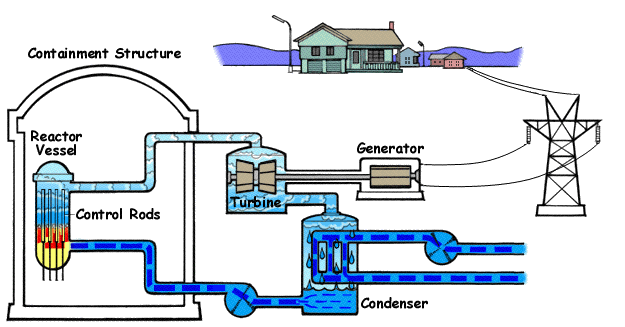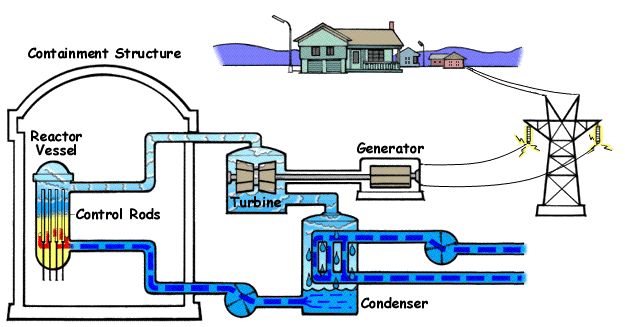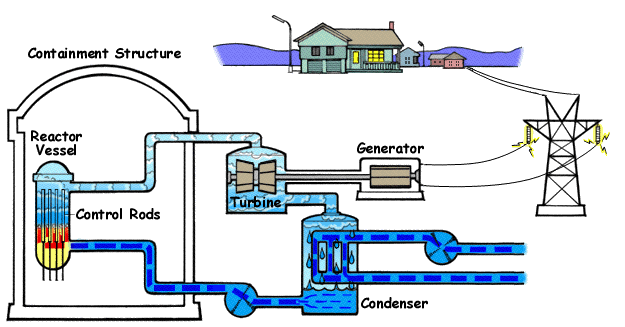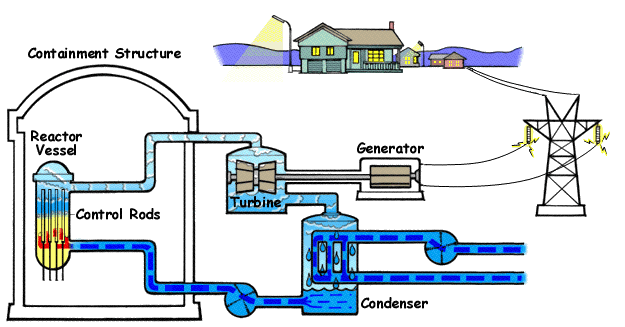
The steps in the Rankine Cycle as shown in Figure 1 and the corresponding steps in the pressure volume diagram (figure 2) are outlined below: [1]
- Pump: Compression of the fluid to high pressure using a pump (this takes work) (Figure 2: Steps 3 to 4)
- Boiler: The compressed fluid is heated to the final temperature (which is at boiling point), therefore, a phase change occurs—from liquid to vapor. (Figure 2: Steps 4 to 1)
- Turbine: Expansion of the vapor in the turbine. (Figure 2: Steps 1 to 2)
- Condenser: Condensation of the vapor in the condenser (where the waste heat goes to the final heat sink (the atmosphere or a large body of water (ex. lake or river). (Figure 2: Steps 2 to 3)
The efficiency of the Rankine cycle is limited by the high heat of vaporization by the fluid. The fluid must be cycled through and reused constantly, therefore, water is the most practical fluid for this cycle. This is not why many power plants are located near a body of water—that's for the waste heat.
As the water condenses in the condenser, waste heat is given off in the form of water vapour—which can be seen billowing from a plant's cooling towers. This waste heat is necessary in any thermodynamic cycle. Due to this condensation step, the pressure at the turbine outlet is lowered. This means the pump requires less work to compress the water—resulting in higher overall efficiencies.
For Further Reading
- Power plant
- Working fluid
- Brayton cycle
- Waste heat
- Efficiency
- Pressure volume diagram
- Or explore a random page
References
- ↑ 1.0 1.1 L. Albright, L. Angenent, F. Vanek. (May 6, 2015). "Stationary Combustion Systems" in Energy Systems Engineering, 2nd Edition. McGraw Hill. 2012. ch.6, pp.161-167
- ↑ Made internally be a member of the Energy Education team, adapted from Energy Systems Engineering by F. Vanek, L. Albright and L. Angenent
- ↑ Wikimedia Commons [Online], Available: https://commons.wikimedia.org/wiki/File:CR-Prozess_p-v-Diagramm.svg
- ↑ USNRC. (June 1, 2015). The Boiling Water Reactor [Online]. Available: http://www.nrc.gov/reading-rm/basic-ref/students/animated-bwr.html

