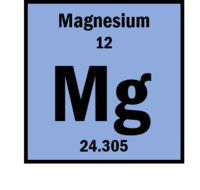Magnesium
Magnesium is the 12th element on the periodic table of elements and it is the eighth most abundant element in the earth's crust.[2] Some of its properties are listed below:[2]
| Atomic weight | 24.305 |
| Density (at 0oC) | 1.74 g/cm3 |
| Boiling point | 1363 K |
| Melting point | 923 K |
Magnesium is an alkaline Earth metal and it is naturally found in rocks and minerals.
Magnesium is an important element for humans, animals, and plants. Humans and animals need it for many of their enzymes to function properly and to maintain healthy bones. Plants contain magnesium as the central molecule in chlorophyll, the compound that makes plants green.[3]
Magnesium Uses

Magnesium is used as an alloying agent with other metals. It is strong and lightweight, so it improves the mechanical characteristics of alloys without drastically increasing the weight. It is not used on its own or in large amounts in compounds because it has a tendency to corrode and combust[2]. Magnesium alloys are used in aircrafts, automotives, and many electronic goods, such as cell phones and computers.[3]
Ignited pure magnesium burns very brightly and easily, so it is used in fireworks and flares (Figure 2). In contrast to this, magnesium oxides and hydroxides are used to to increase the heat and fire resistance of plastics and building materials.[3]
Isotopes
Magnesium has three isotopes found in nature:[2]
| Symbol | Natural Abundance |
|---|---|
| 24Mg | 78.99% |
| 25Mg | 10% |
| 26Mg | 11.01% |
Video
The video below is from the University of Nottingham's periodic videos project.[5] They have created a complete suite of short videos on every element on the periodic table of elements.
For Further Reading
- Periodic table of elements
- Metal
- Alloy
- Combustion
- Or explore a random page
References
- ↑ Made internally by a member of the Energy Education team, with information from periodictable.com, Available: http://periodictable.com/Elements/001/index.html
- ↑ 2.0 2.1 2.2 2.3 Royal Society of Chemistry Periodic Table, Magnesium [Online], Available: http://www.rsc.org/periodic-table/element/12/magnesium
- ↑ 3.0 3.1 3.2 John Emsley, "Nature’s Building Blocks: An A-Z Guide to the Elements", Oxford University Press, New York, 2nd Edition, 2011.
- ↑ Chemistry World,"Podcasts: Magnesium Oxides", Accessed on Oct.8, 2018, Available: https://www.chemistryworld.com/podcasts/magnesium-oxide/7645.article
- ↑ See more videos from the University of Nottingham on different elements here: http://www.periodicvideos.com/