Acid rain
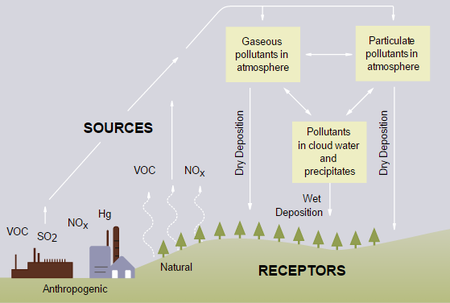
Acid deposition is any type of precipitation - rain, snow, sleet, hail, or fog - that has a lower pH (and is therefore more acidic) than normal. However, the term almost always used for all of these is acid rain. This higher acidity can cause problems in ecosystems and the environment, and remains one of the major environmental concerns from fuel use despite attempts to address the issue since the 1970s.[2]
Acid rain is produced when water in the air combines with nitrogen oxides and sulfur dioxide, two types of pollutants, and then falls down the surface of the Earth. These pollutants may also collect on the Earth's surface and the rain may combine with it upon arrival to the Earth, so the term "acid deposition" is often preferred over acid rain.[2]
Acidity
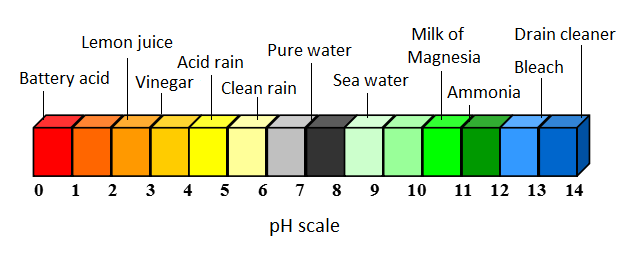
Normal rain has a pH of around 5.6 so it is slightly acidic. this is due to the combination of carbon dioxide with the water in the air to form carbonic acid [3]. This acidity is naturally neutralized when precipitation comes in contact with alkaline minerals such as calcium, magnesium, and potassium in rocks on Earth's surface. This acid neutralization process is behind the chemical weathering of rocks and is responsible for releasing bicarbonates into the soil and water systems, which then act as buffers against stronger acidic inputs.[2] Acid rain, however, has a pH of around 4.2. This means that acid rain is approximately 25 times more acidic than normal rain due to the logarithmic nature of the pH scale.
Impacts
Acid rain has many detrimental effects on vegetation, freshwater ecosystems, and natural and man-made structures. It can also cause respiratory diseases in humans, especially those that have preexisting conditions.[5] When lakes and other bodies of water become too acidic, typically less than a pH of 6.0, plants and aquatic life begin to suffer.[2] Fish reproduction ability falters, with death or deformity being widespread among younger fish. Amphibians and invertebrates suffer similarly, which means that acidic lakes have very little to no animal life.[6]
The most notable impacts have been measured in northeastern sections of North America and Western Europe, with the sources of acidification being traced primarily to coal-fired power plants which emit large amounts of NOx and SO2.[7] The acidity of precipitation can be seen in Figure 3 in the United States, and due to a combination of wind flows from the US and the production of pollutants within Canada, Eastern Canada also suffers from acid rain[8]. Various regulations and restrictions of pollutants have been implemented as a means of addressing the issue, and these power plants must use air pollution control devices to meet them.
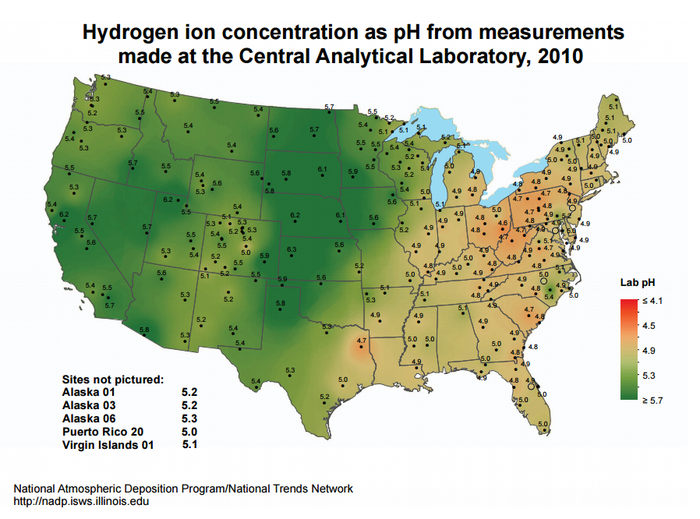
After acid rain falls to the Earth, the effects depend on the type of rock and soil it encounters. Limestone is able to neutralize the acid content of the rain, while granitic bedrocks consisting of granite, quartz, and gneiss are not able to do so as easily[8]. The bedrock in the northeastern regions of Figure 2 is dominated by these granitic rock-types and is overlain only by thin soil layers which means neutralization of acidic precipitation is slow or nonexistent.[7] Lakes at higher elevations in these regions tend to be more acidic, and are considered "dead" if they are not able to support life.
The build up of acid snow on mountains is also a negative consequence, especially since a large amount of the acidic content may be released all at once during the spring in mountain runoff. This chemical shock is devastating to plant life downstream.[7] Many high mountain lakes have been known to lose their fish populations due to increased acidity from snowmelt runoff[8].

Acid Rock Drainage
If preipitation falls onto rocks that contain sulfur bearing minerals such as Pyrite or Galena, the combination of microorganism metabolism and oxidizing atmosphere can lead to runoff with pH levels below three which is over 100 times more acidic than traditional precipitation[11]. This type of acid runoff is commonly found at old abandoned mine sites where sulfur-bearing minerals were previously mined for industrial and economic use. As a result this type of acid deposition is sometimes called Acid Mine Drainage. When the pH of these runoff waters rises above three as runoff is slowly neutralized by carbonates in the environment, the previously dissolved Iron precipitates out as Iron-Oxide compounds. This gives the waters a characteristic yellow-orange colouration known as Yellow boy.
Prevention
Following the realization of the impacts of acid rain, many programs to limit pollution have been put in place around the world since the 1980s[12]. The emissions of SO2 have decreased by 40% in the United States, with acid rain levels down about 65% in 2005. The same is true for NOx.[13] This is made possible by the use of air pollution control devices, such as scrubbers and electrostatic precipitators. Coal power plants and other industrial facilities require these devices in order to meet restrictions on emissions, and can reduce the emissions of some harmful pollutants by 99%[13].
For Further Reading
- Acid
- Base
- The pH scale
- Chemical
- Water
- Water cycle
- Or explore a random page
References
- ↑ Wikimedia Commons [Online], AVailable: https://upload.wikimedia.org/wikipedia/commons/b/b1/Origins_of_acid_rain.svg
- ↑ 2.0 2.1 2.2 2.3 Pollution Probe. The Acid Rain Primer. Visit www.pollutionprobe.org
- ↑ USEPA (May 30, 2020). Acid Rain Students Site [Online]. Accessible: https://www3.epa.gov/acidrain/education/site_students/phscale.html
- ↑ Adapted from Energy: Its use and the Environment. See Reference 8
- ↑ B. Everett, G. Boyle, S. Peake and J. Ramage, "Penalties: Assessing the Environmental and Health Impacts of Energy Use," in Energy Systems and Sustainability, 2nd ed., Oxford, UK: Oxford, 2013, ch.13, pp.543
- ↑ R. Wolfson, "Air Pollution" in Energy, Environment, and Climate, 2nd ed., New York, NY: W.W. Norton & Company, 2012, ch. 6, sec. 2, pp. 129-142
- ↑ 7.0 7.1 7.2 J. Kraushaar and R. Ristinen, "Acid Rain," in Energy and Problems of a Technical Society, 2nd ed., Hoboken, NJ: Wiley, 1993, ch.14, sec.6, pp.404-407
- ↑ 8.0 8.1 8.2 Environment and Climate Change Canada (May 31, 2020). "Acid Rain FAQ" [Online]. Accessible: https://www.ec.gc.ca/air/default.asp?lang=En&n=7E5E9F00-1
- ↑ EPA. Acid Rain in New England [Online], Available: http://www.epa.gov/region1/eco/acidrain/intro.html
- ↑ Wikimedia Commons (June 1, 2020). [Online]. Accessible: https://commons.wikimedia.org/wiki/File:PA_AcidMineDrainage.jpg
- ↑ Warren, L.A. (2011). "Acid Rock Drainage". In: Reitner J., Thiel V. (eds) Encyclopedia of Geobiology. Encyclopedia of Earth Sciences Series. Springer, Dordrecht. [Online]. Accessible: https://link.springer.com/referenceworkentry/10.1007%2F978-1-4020-9212-1_3
- ↑ Nunez, Christina (June 1st, 2020). "Acid Rain, Explained" [Onine]. Accessible: https://www.nationalgeographic.com/environment/global-warming/acid-rain/
- ↑ 13.0 13.1 R. A. Hinrichs and M. Kleinbach, "Acid Rain," in Energy: Its Use and the Environment, 5th ed. Toronto, Ont. Canada: Brooks/Cole, 2006, ch.8, sec.C, pp.252-256

