Gauge pressure: Difference between revisions
energy>Jmdonev No edit summary |
m (1 revision imported) |
(No difference)
| |
Revision as of 22:23, 3 September 2018
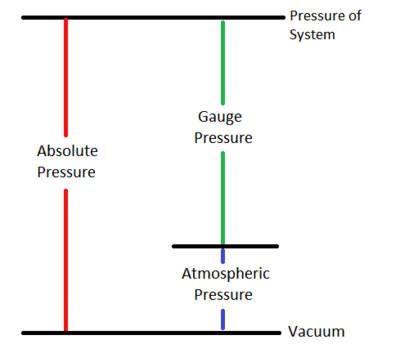
Gauge pressure is a measurement of pressure relative to atmospheric pressure. For this measure of pressure, the zero point is set to be at 1 atm. The gauge pressure is given by the equation:[1]
Where:
is the system's pressure (or absolute pressure),
is atmospheric pressure and
is the gauge pressure.
This relationship is shown in Figure 1. Gauge pressure is often used in everyday situations. For example, tire pressure is measured relative to atmospheric pressure. When a car drives up a mountain the gauge pressure goes up as the atmospheric pressure decreases, but the absolute pressure of the tire remains unchanged assuming the tire does not leak at all.
Gauge pressure should not be used for calculations in the ideal gas law since it's not a true pressure, but rather a pressure difference.
For Further Reading
- Pressure
- Ideal gas law
- Heat engine
- Absolute zero
- Or explore a random page
References
- ↑ The Engineering Toolbox. (April 4, 2015). Pressure [Online]. Available: http://www.engineeringtoolbox.com/pressure-d_587.html