Ionization: Difference between revisions
J.williams (talk | contribs) m (1 revision imported) |
energy>Jmdonev No edit summary |
||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2018-08-03]] | ||
[[File:IONIZATION.png|400px|framed|right|Figure 1. Simplified ionization process where an electron is removed from an atom.<ref>''Created internally by a member of the Energy Education team.''</ref>]] | [[File:IONIZATION.png|400px|framed|right|Figure 1. Simplified ionization process where an electron is removed from an atom.<ref>''Created internally by a member of the Energy Education team.''</ref>]] | ||
<onlyinclude>'''Ionization''' is the process | <onlyinclude>'''Ionization''' is the process by which [[ion]]s are formed by gain or loss of an [[electron]] from an atom or molecule.</onlyinclude><ref> IUPAC. (May 14, 2015). ''Compendium of Chemical Terminology'' [Online], 2nd ed. (the "Gold Book"),2006,"Ionization". Available: http://goldbook.iupac.org/I03183.html</ref> If an atom or molecule gains an electron, it becomes negatively charged (an <em>anion</em>), and if it loses an electron, it becomes positively charged (a <em> cation</em>). Energy may be lost or gained in the formation of an ion. | ||
==Ionization of Atoms== | |||
When an atom gains an electron, energy is usually released. This energy is called the <em>electron affinity</em> of that atomic species. Atoms that have a large electron affinity are more likely to gain an electron and form negative ions.<ref name=libretextEA>Chemistry LibreText. (July 31, 2018). ''Electron Affinity'' [Online]. Available: https://chem.libretexts.org/Textbook_Maps/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Electron_Affinity </ref> | |||
Loss of an electron from an atom requires energy input. The energy needed to remove an electron from a neutral atom is the <em>ionization energy</em> of that atom. It is easier to remove electrons from atoms with a small ionization energy, so they will form [[ion|cations]] more often in [[chemical reaction]]s. <ref name=libretextIE>Chemistry LibreText. (July 31, 2018). ''Ionization Energy'' [Online]. Available: https://chem.libretexts.org/Textbook_Maps/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Ionization_Energy </ref> [[Metal]]s tend to have a smaller ionization energy, and alkali metals (with their single valence electron) have the lowest ionization energy as a group. Thus, we most often find alkali metals as positive ions in chemical compounds - like the sodium cation <chem>Na+</chem> in table salt, NaCl. | |||
Ionization energy is also related to the <em>work function</em> of a metal - the minimum energy needed to eject electrons from a metal surface. The work function of a metal is important in electronics and in creating scientific instruments such as electron guns. Read more about work functions and the photoelectric effect on metals [https://chem.libretexts.org/Textbook_Maps/Physical_and_Theoretical_Chemistry_Textbook_Maps/Book%3A_Quantum_States_of_Atoms_and_Molecules_(Zielinksi_et_al.)/02._Foundations_of_Quantum_Mechanics/2.2%3A_Photoelectric_Effect here.] | |||
The trends in ionization energy and electron affinity, combined with effects from the [[valence_and_core_electrons | electron structure]] of an atom, influence the type and strength of [[chemical bond]]s that form between atoms.<ref>Hyperphysics. (May 19, 2015). ''Ionization Energy'' [Online]. Available: http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/ionize.html</ref> | |||
==Ionizing Radiation== | ==Ionizing Radiation== | ||
:: [[Ionizing radiation|''Main Page'']] | :: [[Ionizing radiation|''Main Page'']] | ||
[[Radiation]] can be classified as "ionizing" if it has enough energy to eject an electron from | [[Radiation]] can be classified as "ionizing" if it has enough energy to eject an electron from an atom. The energies of [[alpha decay|alpha]] and [[beta decay]] particles and [[gamma decay|gamma ray]] [[photon]]s are higher than the ionization energies of most atoms and molecules, so when these types of radiation collide with an atom or molecule, electrons are removed, creating a positive ion (cation). For molecules, exposure to ionizing radiation may also break chemical bonds, fragmenting the molecule. <ref name=harris>Daniel C. Harris, ''Quantitative Chemical Analysis,'' 8th edition. New York, USA: W.H. Freeman and Company, 2010, pg. 504</ref> Since these types of radiation ionize the atoms and molecules they interact with, they are collectively known as ''ionizing radiation''. Ionizing radiation is used to create the ions used in mass spectrometry,<ref name=harris/> a powerful technique for identifying chemical compounds. It is also the basis of the operation of a Geiger counter, which emits "clicks" for every particle of ionizing radiation detected. <ref name=siegel> Peter Siegel. (July 31, 2018). "Introduction to Geiger Counters", ''Phy432 Lab Manual,'' [Online]. California State Polytechnic University. Available: https://www.cpp.edu/~pbsiegel/phy432/labman/geiger.pdf</ref> | ||
==For Further reading== | |||
*[[Electrons and Orbitals]] | |||
*[[Charge]] | |||
*[[Photon]] | |||
*[[Gamma decay]] | |||
*[[Alpha decay]] | |||
*[[Radiation]] | |||
*Or explore a [[Special:Random|random page]] | |||
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
Revision as of 21:49, 12 August 2018
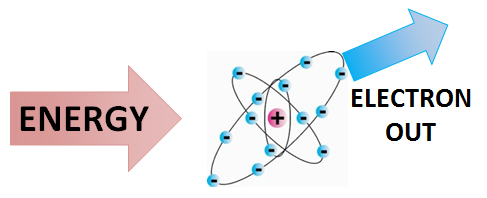
Ionization is the process by which ions are formed by gain or loss of an electron from an atom or molecule.[2] If an atom or molecule gains an electron, it becomes negatively charged (an anion), and if it loses an electron, it becomes positively charged (a cation). Energy may be lost or gained in the formation of an ion.
Ionization of Atoms
When an atom gains an electron, energy is usually released. This energy is called the electron affinity of that atomic species. Atoms that have a large electron affinity are more likely to gain an electron and form negative ions.[3]
Loss of an electron from an atom requires energy input. The energy needed to remove an electron from a neutral atom is the ionization energy of that atom. It is easier to remove electrons from atoms with a small ionization energy, so they will form cations more often in chemical reactions. [4] Metals tend to have a smaller ionization energy, and alkali metals (with their single valence electron) have the lowest ionization energy as a group. Thus, we most often find alkali metals as positive ions in chemical compounds - like the sodium cation in table salt, NaCl.
Ionization energy is also related to the work function of a metal - the minimum energy needed to eject electrons from a metal surface. The work function of a metal is important in electronics and in creating scientific instruments such as electron guns. Read more about work functions and the photoelectric effect on metals here.
The trends in ionization energy and electron affinity, combined with effects from the electron structure of an atom, influence the type and strength of chemical bonds that form between atoms.[5]
Ionizing Radiation
Radiation can be classified as "ionizing" if it has enough energy to eject an electron from an atom. The energies of alpha and beta decay particles and gamma ray photons are higher than the ionization energies of most atoms and molecules, so when these types of radiation collide with an atom or molecule, electrons are removed, creating a positive ion (cation). For molecules, exposure to ionizing radiation may also break chemical bonds, fragmenting the molecule. [6] Since these types of radiation ionize the atoms and molecules they interact with, they are collectively known as ionizing radiation. Ionizing radiation is used to create the ions used in mass spectrometry,[6] a powerful technique for identifying chemical compounds. It is also the basis of the operation of a Geiger counter, which emits "clicks" for every particle of ionizing radiation detected. [7]
For Further reading
- Electrons and Orbitals
- Charge
- Photon
- Gamma decay
- Alpha decay
- Radiation
- Or explore a random page
References
- ↑ Created internally by a member of the Energy Education team.
- ↑ IUPAC. (May 14, 2015). Compendium of Chemical Terminology [Online], 2nd ed. (the "Gold Book"),2006,"Ionization". Available: http://goldbook.iupac.org/I03183.html
- ↑ Chemistry LibreText. (July 31, 2018). Electron Affinity [Online]. Available: https://chem.libretexts.org/Textbook_Maps/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Electron_Affinity
- ↑ Chemistry LibreText. (July 31, 2018). Ionization Energy [Online]. Available: https://chem.libretexts.org/Textbook_Maps/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Ionization_Energy
- ↑ Hyperphysics. (May 19, 2015). Ionization Energy [Online]. Available: http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/ionize.html
- ↑ 6.0 6.1 Daniel C. Harris, Quantitative Chemical Analysis, 8th edition. New York, USA: W.H. Freeman and Company, 2010, pg. 504
- ↑ Peter Siegel. (July 31, 2018). "Introduction to Geiger Counters", Phy432 Lab Manual, [Online]. California State Polytechnic University. Available: https://www.cpp.edu/~pbsiegel/phy432/labman/geiger.pdf

