Ionizing radiation: Difference between revisions
J.williams (talk | contribs) m (1 revision imported) |
energy>Jmdonev No edit summary |
||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2018-08-03]] | ||
[[File:IONIZATION.png|400px|framed|right|Figure 1. Ionizing radiation is radiation that can strip electrons from atoms. This process is shown above.<ref>''Created internally by a member of the Energy Education team.''</ref>]] | [[File:IONIZATION.png|400px|framed|right|Figure 1. Ionizing radiation is radiation that can strip electrons from atoms. This process is shown above.<ref>''Created internally by a member of the Energy Education team.''</ref>]] | ||
<onlyinclude>'''Ionizing radiation''' is a specific type of [[radiation]] that has enough [[energy]] to eject an [[electron]] from some [[atom]]. Generally speaking, the energies of [[alpha decay|alpha]] and [[beta decay]] particles and [[gamma decay|gamma ray]] [[photon]]s is higher than the [[ionization]] energies of atoms and molecules.</onlyinclude> This means that instead of being absorbed, these particles ionize the matter and break molecular bonds. These resulting particles are therefore known as ionizing radiation.<ref name=phys>R. Knight. (May 20, 2015). ''Physics for Scientists and Engineers'', 3rd ed. U.S.A.: Pearson</ref> Ionizing radiation is important as it can produce a number of physiological side-effects, such as cancer.<ref name="RE1">Hyperphysics. (May 19, 2015). ''Ionizing Radiation'' [Online]. Available: http://hyperphysics.phy-astr.gsu.edu/hbase/mod4.html</ref> | <onlyinclude>'''Ionizing radiation''' is a specific type of [[radiation]] that has enough [[energy]] to eject an [[electron]] from some [[atom]]. Generally speaking, the energies of [[alpha decay|alpha]] and [[beta decay]] particles and [[gamma decay|gamma ray]] [[photon]]s is higher than the [[ionization]] energies of atoms and molecules.</onlyinclude> This means that instead of being absorbed, these particles ionize the matter and break molecular bonds. These resulting particles are therefore known as ionizing radiation.<ref name=phys>R. Knight. (May 20, 2015). ''Physics for Scientists and Engineers'', 3rd ed. U.S.A.: Pearson</ref> Ionizing radiation is important as it can produce a number of physiological side-effects, such as cancer.<ref name="RE1">Hyperphysics. (May 19, 2015). ''Ionizing Radiation'' [Online]. Available: http://hyperphysics.phy-astr.gsu.edu/hbase/mod4.html</ref> | ||
The idea that radiation can be ionizing is the basis | The idea that radiation can be ionizing is the basis of operating the Geiger counter—one detector of nuclear radiation. Geiger counters only detect ionizing radiation as they rely on the products of ionization to operate. | ||
==Uses of Ionizing Radiation== | ==Uses of Ionizing Radiation== | ||
Different forms of ionizing radiation have different uses. Radiotherapy, a cancer treatment, relies on beta decay and uses its ionizing properties to kill cancer cells.<ref name=" | Different forms of ionizing radiation have different uses. Radiotherapy, a cancer treatment, relies on beta decay and uses its ionizing properties to kill cancer cells.<ref name="RE2">ChemTeacher. (July 22, 2015). ''Beta Decay'' [Online]. Available: http://chemteacher.chemeddl.org/services/chemteacher/index.php?option=com_content&view=article&id=66M</ref> Alpha radiation also has use in the medical field, with targeted alpha therapy being used to kill cancer. | ||
Ionizing radiation also has uses outside of the medical field. The ionizing properties of [[americium]] results in its use in smoke detectors. Inside the smoke detector alpha particles from the americium are released. This in turn [[ionization|ionizes]] the air inside the detector. Smoke in the detector absorbs this alpha radiation, so if smoke is present the ionization is altered and the alarm is triggered.<ref>BBC Bitesized. (July 22, 2015). ''Uses of Radiation'' [Online]. Available: http://www.bbc.co.uk/schools/gcsebitesize/science/ocr_gateway_pre_2011/living_future/4_nuclear_radiation2.shtml</ref> | Ionizing radiation also has uses outside of the medical field. The ionizing properties of [[americium]] results in its use in smoke detectors. Inside the smoke detector alpha particles from the americium are released. This in turn [[ionization|ionizes]] the air inside the detector. Smoke in the detector absorbs this alpha radiation, so if smoke is present the ionization is altered and the alarm is triggered.<ref>BBC Bitesized. (July 22, 2015). ''Uses of Radiation'' [Online]. Available: http://www.bbc.co.uk/schools/gcsebitesize/science/ocr_gateway_pre_2011/living_future/4_nuclear_radiation2.shtml</ref> In addition, cobalt—a source of gamma ionizing radiation—is used to sterilize medical equipment and [[irradiate food]], killing bacteria and pasteurizing the food.<ref name=EPA>US EPA. (May 14, 2015). ''Gamma Rays'' [Online]. Available: http://www.epa.gov/radiation/understand/gamma.html#use</ref> | ||
==Health Effects== | ==Health Effects== | ||
The [[biological effects of radiation]] vary depending on how much exposure a person has, | The [[biological effects of radiation]] vary depending on how much exposure a person has, the length of exposure they are expose to, and what type of radiation they are exposed to. Ionizing radiation is produced when radioactive materials decay, causing damage to living tissues that cannot always be repaired.<ref name="RE3">US EPA. (July 8, 2015). ''Health Effects: Radiation'' [Online]. Available: http://www.epa.gov/radiation/understand/health_effects.html#q1</ref> Chronic exposure to radiation can lead to cancer (as a result of damage at the cellular or molecular level) or other mutations that can be harmful to fetuses. Effects from acute exposure to ionizing radiation appear quickly, and include burns and radiation poisoning. The symptoms of radiation poisoning include nausea, weakness, hair loss, and diminished organ function and this radiation sickness can result in death if the dose is high enough.<ref name="RE3"/> | ||
==For Further Reading== | |||
*[[Radiation]] | |||
*[[Energy]] | |||
*[[Alpha decay]] | |||
*[[Gamma decay]] | |||
*[[Photon]] | |||
*Or explore a [[Special:Random|random page]] | |||
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
[[Category:Uploaded]] | [[Category:Uploaded]] | ||
Revision as of 20:21, 31 July 2018
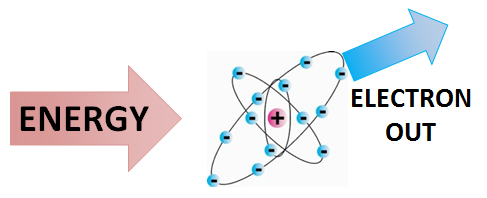
Ionizing radiation is a specific type of radiation that has enough energy to eject an electron from some atom. Generally speaking, the energies of alpha and beta decay particles and gamma ray photons is higher than the ionization energies of atoms and molecules. This means that instead of being absorbed, these particles ionize the matter and break molecular bonds. These resulting particles are therefore known as ionizing radiation.[2] Ionizing radiation is important as it can produce a number of physiological side-effects, such as cancer.[3]
The idea that radiation can be ionizing is the basis of operating the Geiger counter—one detector of nuclear radiation. Geiger counters only detect ionizing radiation as they rely on the products of ionization to operate.
Uses of Ionizing Radiation
Different forms of ionizing radiation have different uses. Radiotherapy, a cancer treatment, relies on beta decay and uses its ionizing properties to kill cancer cells.[4] Alpha radiation also has use in the medical field, with targeted alpha therapy being used to kill cancer.
Ionizing radiation also has uses outside of the medical field. The ionizing properties of americium results in its use in smoke detectors. Inside the smoke detector alpha particles from the americium are released. This in turn ionizes the air inside the detector. Smoke in the detector absorbs this alpha radiation, so if smoke is present the ionization is altered and the alarm is triggered.[5] In addition, cobalt—a source of gamma ionizing radiation—is used to sterilize medical equipment and irradiate food, killing bacteria and pasteurizing the food.[6]
Health Effects
The biological effects of radiation vary depending on how much exposure a person has, the length of exposure they are expose to, and what type of radiation they are exposed to. Ionizing radiation is produced when radioactive materials decay, causing damage to living tissues that cannot always be repaired.[7] Chronic exposure to radiation can lead to cancer (as a result of damage at the cellular or molecular level) or other mutations that can be harmful to fetuses. Effects from acute exposure to ionizing radiation appear quickly, and include burns and radiation poisoning. The symptoms of radiation poisoning include nausea, weakness, hair loss, and diminished organ function and this radiation sickness can result in death if the dose is high enough.[7]
For Further Reading
- Radiation
- Energy
- Alpha decay
- Gamma decay
- Photon
- Or explore a random page
References
- ↑ Created internally by a member of the Energy Education team.
- ↑ R. Knight. (May 20, 2015). Physics for Scientists and Engineers, 3rd ed. U.S.A.: Pearson
- ↑ Hyperphysics. (May 19, 2015). Ionizing Radiation [Online]. Available: http://hyperphysics.phy-astr.gsu.edu/hbase/mod4.html
- ↑ ChemTeacher. (July 22, 2015). Beta Decay [Online]. Available: http://chemteacher.chemeddl.org/services/chemteacher/index.php?option=com_content&view=article&id=66M
- ↑ BBC Bitesized. (July 22, 2015). Uses of Radiation [Online]. Available: http://www.bbc.co.uk/schools/gcsebitesize/science/ocr_gateway_pre_2011/living_future/4_nuclear_radiation2.shtml
- ↑ US EPA. (May 14, 2015). Gamma Rays [Online]. Available: http://www.epa.gov/radiation/understand/gamma.html#use
- ↑ 7.0 7.1 US EPA. (July 8, 2015). Health Effects: Radiation [Online]. Available: http://www.epa.gov/radiation/understand/health_effects.html#q1

