Proton: Difference between revisions
J.williams (talk | contribs) m (1 revision imported) |
No edit summary |
||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2018-06-15]] [[category:Phets]] | ||
[[File:Nuclear force.png|350px|thumbnail|Figure 1. The electrical force pushing protons apart and the strong force acting on both protons and neutrons inside a nucleus<ref>Prof. Matt Strassler [Online]. Available: http://profmattstrassler.com/articles-and-posts/particle-physics-basics/the-structure-of-matter/the-nuclei-of-atoms-at-the-heart-of-matter/what-holds-nuclei-together/ [Accessed on 24 October 2014]. </ref>]] | [[File:Nuclear force.png|350px|thumbnail|Figure 1. The electrical force pushing protons apart and the strong force acting on both protons and neutrons inside a nucleus<ref>Prof. Matt Strassler [Online]. Available: http://profmattstrassler.com/articles-and-posts/particle-physics-basics/the-structure-of-matter/the-nuclei-of-atoms-at-the-heart-of-matter/what-holds-nuclei-together/ [Accessed on 24 October 2014]. </ref>]] | ||
<onlyinclude>'''Protons''' are the positively [[charge]]d particles that are inside the [[nucleus]] of an [[atom]].</onlyinclude>The protons are pushed apart by the [[electromagnetic force]] but pulled together by the [[strong force]], which is stronger over short distances (these distances are about a [[meter|fm]] or 10<sup>-15</sup> m). Protons are incredibly small, about 10<sup>-15</sup> [[meter|m]], 10,000x smaller than an atom! Please see [[size of the universe]] for some online demonstrations to show this scale. Despite their incredibly small size, protons push against each other with tremendous forces, about 100 [[newton|N]], comparable to the weight of a small dog! | <onlyinclude>'''Protons''' are the positively [[charge]]d particles that are inside the [[nucleus]] of an [[atom]].</onlyinclude>The protons are pushed apart by the [[electromagnetic force]] but pulled together by the [[strong force]], which is stronger over short distances (these distances are about a [[meter|fm]] or 10<sup>-15</sup> m). Protons are incredibly small, about 10<sup>-15</sup> [[meter|m]], 10,000x smaller than an atom!<ref>"Quantum Diaries", Quantumdiaries.org, 2018. [Online]. Available: https://www.quantumdiaries.org/2010/07/12/the-size-of-the-proton/. [Accessed: 12- Jun- 2018].</ref> Please see [[size of the universe]] for some online demonstrations to show this scale. Despite their incredibly small size, protons push against each other with tremendous forces, about 100 [[newton|N]], comparable to the weight of a small dog! | ||
The charge on a proton is exactly equal and opposite to the charge on an [[electron]]. | The charge on a proton is exactly equal and opposite to the charge on an [[electron]]. Therefore, the number of electrons in a neutral atom is always equal to the number of protons (see the bottom of the page for a PhET simulation about that). Protons are made of smaller particles called [http://profmattstrassler.com/articles-and-posts/particle-physics-basics/the-known-apparently-elementary-particles/ quarks] (or see [http://hyperphysics.phy-astr.gsu.edu/hbase/particles/quark.html#c1 hyperphysics]), which also make up [[neutron]]s. | ||
The number of protons in a nucleus is called the [[atomic number]], and this number determines what [[element]] an atom is. In other words, changing the proton number changes the element. This number of protons (atomic number) changes when a nucleus undergoes [[beta decay]] in any of its various forms, | The number of protons in a nucleus is called the [[atomic number]], and this number determines what [[element]] an atom is. In other words, changing the proton number changes the element. This number of protons (atomic number) changes when a nucleus undergoes [[beta decay]] or [[alpha decay]] in any of its various forms.<ref>"17.3: Types of Radioactivity: Alpha, Beta, and Gamma Decay - Chemistry LibreTexts", Chem.libretexts.org, 2018. [Online]. Available: https://chem.libretexts.org/Textbook_Maps/Introductory_Chemistry/Map%3A_Introductory_Chemistry_(Tro)/17%3A_Radioactivity_and_Nuclear_Chemistry/17.03%3A_Types_of_Radioactivity%3A_Alpha%2C_Beta%2C_and_Gamma_Decay. [Accessed: 12- Jun- 2018].</ref> The difficulty with changing the number of protons in a nucleus on purpose is why alchemy (the medieval practice of trying to turn lead into gold) failed for so long! | ||
To learn more about protons please see [http://profmattstrassler.com/articles-and-posts/largehadroncolliderfaq/whats-a-proton-anyway/ What's a proton] by Prof. Matt Strassler or [http://hyperphysics.phy-astr.gsu.edu/hbase/particles/proton.html#c1 hyperphysics]. | To learn more about protons please see [http://profmattstrassler.com/articles-and-posts/largehadroncolliderfaq/whats-a-proton-anyway/ What's a proton] by Prof. Matt Strassler or [http://hyperphysics.phy-astr.gsu.edu/hbase/particles/proton.html#c1 hyperphysics]. | ||
| Line 14: | Line 14: | ||
<html><iframe src="http://phet.colorado.edu/sims/html/build-an-atom/latest/build-an-atom_en.html" width="816" height="459"></iframe></html> | <html><iframe src="http://phet.colorado.edu/sims/html/build-an-atom/latest/build-an-atom_en.html" width="816" height="459"></iframe></html> | ||
==For Further Reading== | |||
*[[Atom]] | |||
*[[Electron]] | |||
*[[Neutron]] | |||
*[[Nucleus]] | |||
*[[Electromagnetic force]] | |||
*[[Alpha decay]] and [[Beta decay]] | |||
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
[[Category:Uploaded]] | [[Category:Uploaded]] | ||
Revision as of 17:21, 13 June 2018
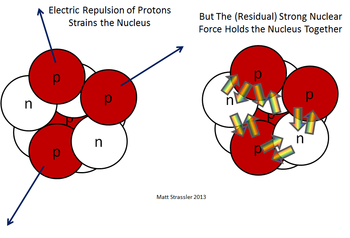
Protons are the positively charged particles that are inside the nucleus of an atom.The protons are pushed apart by the electromagnetic force but pulled together by the strong force, which is stronger over short distances (these distances are about a fm or 10-15 m). Protons are incredibly small, about 10-15 m, 10,000x smaller than an atom![2] Please see size of the universe for some online demonstrations to show this scale. Despite their incredibly small size, protons push against each other with tremendous forces, about 100 N, comparable to the weight of a small dog!
The charge on a proton is exactly equal and opposite to the charge on an electron. Therefore, the number of electrons in a neutral atom is always equal to the number of protons (see the bottom of the page for a PhET simulation about that). Protons are made of smaller particles called quarks (or see hyperphysics), which also make up neutrons.
The number of protons in a nucleus is called the atomic number, and this number determines what element an atom is. In other words, changing the proton number changes the element. This number of protons (atomic number) changes when a nucleus undergoes beta decay or alpha decay in any of its various forms.[3] The difficulty with changing the number of protons in a nucleus on purpose is why alchemy (the medieval practice of trying to turn lead into gold) failed for so long!
To learn more about protons please see What's a proton by Prof. Matt Strassler or hyperphysics.
PhET: Build an Atom
The University of Colorado has graciously allowed us to use the following PhET simulation. This simulation builds atoms from protons, neutrons, and electrons and tests knowledge of the periodic table. The simulation shows how the neutrons and protons must balance for the nucleus to be stable.
For Further Reading
References
- ↑ Prof. Matt Strassler [Online]. Available: http://profmattstrassler.com/articles-and-posts/particle-physics-basics/the-structure-of-matter/the-nuclei-of-atoms-at-the-heart-of-matter/what-holds-nuclei-together/ [Accessed on 24 October 2014].
- ↑ "Quantum Diaries", Quantumdiaries.org, 2018. [Online]. Available: https://www.quantumdiaries.org/2010/07/12/the-size-of-the-proton/. [Accessed: 12- Jun- 2018].
- ↑ "17.3: Types of Radioactivity: Alpha, Beta, and Gamma Decay - Chemistry LibreTexts", Chem.libretexts.org, 2018. [Online]. Available: https://chem.libretexts.org/Textbook_Maps/Introductory_Chemistry/Map%3A_Introductory_Chemistry_(Tro)/17%3A_Radioactivity_and_Nuclear_Chemistry/17.03%3A_Types_of_Radioactivity%3A_Alpha%2C_Beta%2C_and_Gamma_Decay. [Accessed: 12- Jun- 2018].

