Alkene: Difference between revisions
No edit summary |
m (1 revision imported) |
||
| (One intermediate revision by one other user not shown) | |||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2021-01-31]] | ||
<onlyinclude>An '''alkene''' is one of the four main types of [[hydrocarbon]]s. Alkenes must contain at least one carbon to carbon double bond in their chain. Alkenes are true hydrocarbons, meaning they are made up of only [[hydrogen]] and [[carbon]].</onlyinclude><ref>“Alkenes,” Chemistry LibreTexts, 28-Nov-2016. [Online]. Available: http://chemwiki.ucdavis.edu/Organic_Chemistry/Hydrocarbons/Alkenes. [Accessed: 19-May-2017].</ref> | [[Category: Translated to French]] | ||
[[fr:Alcène]] | |||
<onlyinclude>An '''alkene''', also called an '''olefin''', is one of the four main types of [[hydrocarbon]]s. Alkenes must contain at least one carbon to carbon double bond in their chain. Alkenes are true hydrocarbons, meaning they are made up of only [[hydrogen]] and [[carbon]].</onlyinclude><ref>“Alkenes,” Chemistry LibreTexts, 28-Nov-2016. [Online]. Available: http://chemwiki.ucdavis.edu/Organic_Chemistry/Hydrocarbons/Alkenes. [Accessed: 19-May-2017].</ref> | |||
Alkenes have the molecular formula C<sub>'''''n''''' </sub>H<sub>2'''''n'''''</sub>, where: | Alkenes have the molecular formula C<sub>'''''n''''' </sub>H<sub>2'''''n'''''</sub>, where: | ||
| Line 7: | Line 9: | ||
*'''''n''''' refers to the number of carbon [[atom]]s. | *'''''n''''' refers to the number of carbon [[atom]]s. | ||
Alkenes are similar to [[Alkane]]s except they contain a double bond between two carbon atoms. When one carbon shares a double bond with another, this limits the number of hydrogen which can be bonded (resulting in less hydrogen atoms per carbon atom). | |||
Alkenes are similar to [[Alkane]]s except they contain a double bond between two carbon atoms instead of a single bond. When one carbon shares a double bond with another, this limits the number of hydrogen which can be bonded (resulting in less hydrogen atoms per carbon atom). | |||
The two simplest alkenes are [[ethene]] (C<sub>2</sub>H<sub>4</sub>) and [[propene]] (C<sub>3</sub>H<sub>6</sub>). | The two simplest alkenes are [[ethene]] (C<sub>2</sub>H<sub>4</sub>) and [[propene]] (C<sub>3</sub>H<sub>6</sub>). | ||
| Line 16: | Line 19: | ||
</gallery> | </gallery> | ||
Alkanes can be recognized by their '''-ene''' suffix. | |||
When there are 4 or more carbons in a chain, the position of the double bond can create different possible structures and uses a more precise nomenclature. Compounds that have the same chemical formula but have different bonding arrangements are called [[chemical isomer|structural isomers]]. | |||
To learn more about alkenes, click [http://chemwiki.ucdavis.edu/Organic_Chemistry/Hydrocarbons/Alkenes here]. | To learn more about alkenes, click [http://chemwiki.ucdavis.edu/Organic_Chemistry/Hydrocarbons/Alkenes here]. | ||
==Further Reading== | |||
*[[Alkane]] | |||
*[[Alkyne]] | |||
*[[Hydrocarbon]] | |||
*Or explore a [[Special:Random|random page]] | |||
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
[[Category:Uploaded]] | [[Category:Uploaded]] | ||
Latest revision as of 00:02, 27 September 2021
An alkene, also called an olefin, is one of the four main types of hydrocarbons. Alkenes must contain at least one carbon to carbon double bond in their chain. Alkenes are true hydrocarbons, meaning they are made up of only hydrogen and carbon.[1]
Alkenes have the molecular formula Cn H2n, where:
- C is Carbon.
- H is Hydrogen.
- n refers to the number of carbon atoms.
Alkenes are similar to Alkanes except they contain a double bond between two carbon atoms instead of a single bond. When one carbon shares a double bond with another, this limits the number of hydrogen which can be bonded (resulting in less hydrogen atoms per carbon atom).
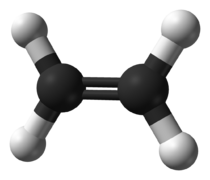
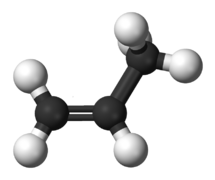
The two simplest alkenes are ethene (C2H4) and propene (C3H6).
Alkanes can be recognized by their -ene suffix.
When there are 4 or more carbons in a chain, the position of the double bond can create different possible structures and uses a more precise nomenclature. Compounds that have the same chemical formula but have different bonding arrangements are called structural isomers.
To learn more about alkenes, click here.
Further Reading
- Alkane
- Alkyne
- Hydrocarbon
- Or explore a random page
References
- ↑ “Alkenes,” Chemistry LibreTexts, 28-Nov-2016. [Online]. Available: http://chemwiki.ucdavis.edu/Organic_Chemistry/Hydrocarbons/Alkenes. [Accessed: 19-May-2017].
- ↑ https://commons.wikimedia.org/wiki/File:Ethylene-CRC-MW-3D-balls.png
- ↑ https://commons.wikimedia.org/wiki/File:Propylene-3D-balls.png