Atom
An atom is the most basic unit of an element - all elements have distinct properties because of the structure of their atoms. For example, an atom on the surface of a silicon crystal (see Figure 1) will be different from those on the surface of a uranium crystal. The word 'atom' comes from the Greek roots 'a' (without) and 'tom' (to cut).[3] Up until the 20th century, atoms were believed to be the smallest possible particle.
Composition
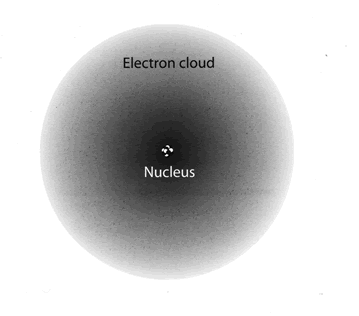
The nucleus is the central, highly dense component of an atom (see figure 2). It is composed of protons and neutrons (collectively called nucleons) and is responsible for the large majority of the atomic mass. Protons and neutrons are held together in the nucleus by what's called the strong nuclear force (which is the strongest known force in the universe). Surrounding the nucleus is a cloud of much smaller and lighter electrons, which are attracted to the nucleus by the electromagnetic force from interacting with the protons. Differing quantities of protons, neutrons, and electrons cause the atom to have differing chemical properties, which determine what element that atom is.
Atoms are unimaginably small, and their nuclei are 1000 times smaller. In fact, one cubic centimeter of the silicon seen in figure 1 contains approximately 5 x 1022 atoms (that's 5 with 22 zeros after it!). Please check out the scale of the universe to see a visual representation of just how small atoms are.
Identifying the Atom
Protons
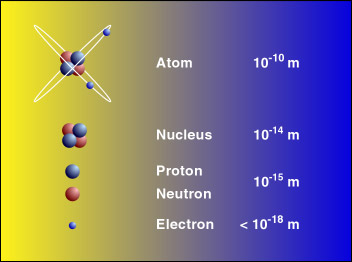
An element is identifiable by the number of protons found within a nucleus of one of its atoms (see figure 3); furthermore, the number of protons in the atom also determines the element's location on the periodic table of elements. For example, a carbon atom has exactly 6 protons in its nucleus and is thus number 6 on the periodic table of elements, thorium has exactly 90 protons and is thus number 90 on the periodic table of elements.
Electrons
Atoms have an equal number of protons and electrons; however, an atom can lose or gain electron(s) becoming "unbalanced." An unbalanced atom is called an ion; if it gains an electron (thus having more electrons than protons) it becomes a negatively charged ion or an anion. If the opposite happens and the atom loses an electron it becomes a positively charged ion or a cation. Ions can bond readily to other ions, creating a wide variety of different compounds.
One way that atoms gain or lose electrons is with high energy radiation. This radiation causes ions to form and as a result is called ionizing radiation.
Neutrons
Neutrons have the same mass as protons, making it very easy to determine how many are within a nucleus of an atom. Simply subtracting the number of protons from the atomic mass of the atom will give the number of neutrons. For example, cesium is number 55 on the periodic table of elements and thus has 55 protons; furthermore, its atomic mass (typically also found on the periodic table) is known to be 133 amu (atomic mass units). Subtracting 55 from 133 gives 78, which is thus the number of neutrons within the atom. The same type of atom (determined by number of protons) can have different numbers of neutrons. These are referred to as different isotopes of an atom. For example carbon-12 (12C) is one isotope of carbon and carbon-14 (14C) is another isotope of carbon. This is discussed more on the page about the nucleus.
Knowing Nuclear
Our team has created a video in the Knowing Nuclear stream about this:
Phet: Build an Atom
Below is a interactive PhET simulation from the University of Colorado. This simulation creates an atom from protons, neutrons, and electrons and test skills with the periodic table.
For more information about atoms, please see UC Davis's chem wiki.
References
- ↑ For an explanation of these amazing microscopes please see hyperphysics
- ↑ Downloaded from http://phys.org/news70293607.html
- ↑ The Compact Oxford English dictionary pg 84, Oxford University Press, 2004.
- ↑ "The electron cloud" internet: http://letstalkaboutscience.wordpress.com/2012/02/16/the-electron-cloud/
- ↑ "Nature of the Universe: Chapter Eleven" internet:http://www.lcsd.gov.hk/CE/Museum/Space/EducationResource/Universe/framed_e/lecture/ch11/ch11.html


