Proton
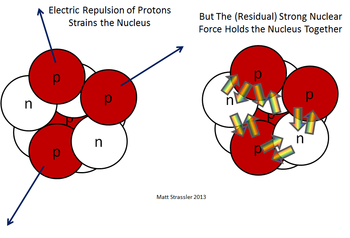
Protons are the positively charged particles that are inside the nucleus of an atom. The protons are pushed apart by the electromagnetic force but pulled together by the strong force, which is stronger over short distances (these distances are about a fm or 10-15 m). Protons are incredibly small, about 10-15 m, 10,000x smaller than an atom![2] Despite their incredibly small size, protons push against each other with tremendous forces, about 100 N, comparable to the weight of a small dog!
Please see size of the universe for some online demonstrations to show the scale of protons.
The charge on a proton is exactly equal and opposite to the charge on an electron. Therefore, the number of electrons in a neutral atom is always equal to the number of protons (click here for a Phet simulation about that). Protons are made of smaller particles called quarks (or see hyperphysics for more on quarks), which also make up neutrons.
The number of protons in a nucleus is called the atomic number, and this number determines the element an atom is. In other words, changing the proton number changes the element. This number of protons (the atomic number) changes when a nucleus undergoes beta decay or alpha decay in any of its various forms.[3] The difficulty with changing the number of protons in a nucleus on purpose is why alchemy (which is the medieval practice of trying to turn lead into gold) failed for so long!
To learn more about protons please see What's a proton by Prof. Matt Strassler or hyperphysics.
Phet: Build a Nucleus
The Energy education team has adapted the following simulation from the University of Colorado. This simulation shows how neutrons and protons sit in energy levels and make up the nucleus. The number of neutrons and protons maintain particular ratios for the nucleus to be stable.
For Further Reading
References
- ↑ Prof. Matt Strassler [Online]. Available: http://profmattstrassler.com/articles-and-posts/particle-physics-basics/the-structure-of-matter/the-nuclei-of-atoms-at-the-heart-of-matter/what-holds-nuclei-together/ [Accessed on 24 October 2014].
- ↑ "Quantum Diaries", Quantumdiaries.org, 2018. [Online]. Available: https://www.quantumdiaries.org/2010/07/12/the-size-of-the-proton/. [Accessed: 12- Jun- 2018].
- ↑ "17.3: Types of Radioactivity: Alpha, Beta, and Gamma Decay - Chemistry LibreTexts", Chem.libretexts.org, 2018. [Online]. Available: https://chem.libretexts.org/Textbook_Maps/Introductory_Chemistry/Map%3A_Introductory_Chemistry_(Tro)/17%3A_Radioactivity_and_Nuclear_Chemistry/17.03%3A_Types_of_Radioactivity%3A_Alpha%2C_Beta%2C_and_Gamma_Decay. [Accessed: 12- Jun- 2018].

