Alpha decay
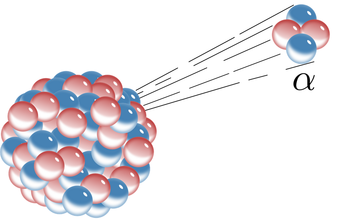
Alpha decay is a nuclear decay process where an unstable nucleus changes to another element by shooting out a particle composed of two protons and two neutrons.[2] This ejected particle is known as an alpha particle and is simply a helium nucleus. Alpha particles have a relatively large mass and a positive charge. This large mass means alpha particles can't go very far through the air, or get very deep into a solid. Alpha particles only affect surfaces, so alpha decay is rarely used in external medical radiation therapy.[3]
Alpha decay was originally distinguished from other forms of radiation by Ernest Rutherford by observing the deflection of the radiation through a magnetic field. Alpha decay deflects in the way you would expect a positive particle to since alpha particles have a charge.[4]
The general equation representing alpha decay is:
where:
- is the parent nucleus, the starting nucleus.
- Is the total number of nucleons (the number of neutrons plus the number of protons).
- Is the total number of protons.
- is the daughter nucleus, the ending nucleus
- is the released alpha particle
Safety
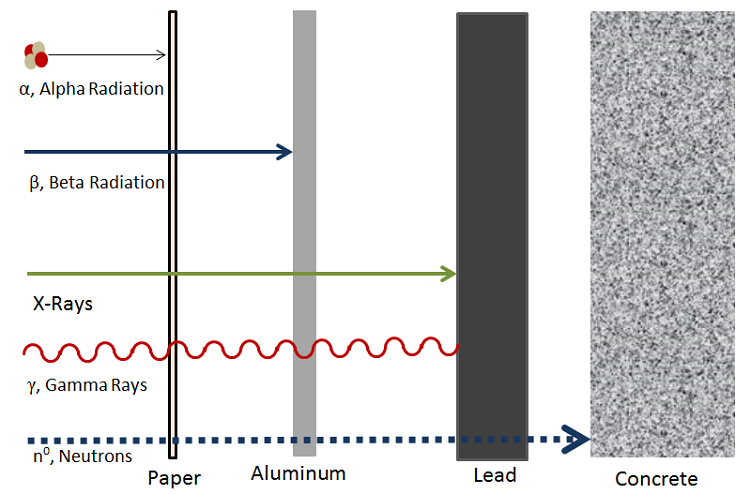
Although not very penetrating, the ingestion of a substance that undergoes alpha decay is harmful as the ejected alpha particles can damage internal tissues very easily despite its short range. This damage is a result of contact with membranes and living cells.[3] Figure 2 shows a diagram that illustrates the different levels of penetration for different types of radiation.
Overall, the health effects of alpha particles vary with how the exposure takes place. If the alpha emitter is inhaled, swallowed, or absorbed into the blood stream there can be lasting biological damage. This damage increases a persons risk of cancer. Alpha radiation is known to cause lung cancer in humans if the alpha emitter is inhaled. The inhalation of radon, an alpha emitter, is one of the biggest sources of alpha decay related illness in humans.[7]
Applications and Importance

Radioactive elements that undergo alpha decay are used in smoke detectors (see Figure 3). Americium is one frequently used element as it is a major alpha particle source. Inside the smoke detertor alpha particles are released. This in turn ionizes the air inside the detector. Smoke in the detector absorbs this alpha radiation, so if smoke is present the ionization is altered and the alarm is triggered.[9]
As well, alpha particles are used in a process known as Alpha Particle X-Ray Spectroscopy (APXS). This process is used to determine the elemental composition of rocks and soil. NASA used this method in its missions to Mars, including the Pathfinder missions, to determine what elements existed Martian rocks.[10]
Alpha particles do also have some use in the medical field. A new cancer treatment known as targeted alpha therapy or TAT uses alpha decay to kill cancer cells. Lead-212 is ingested and travels to the site of the tumor, then giving off alpha radiation which kills all the cells in the area.[10]
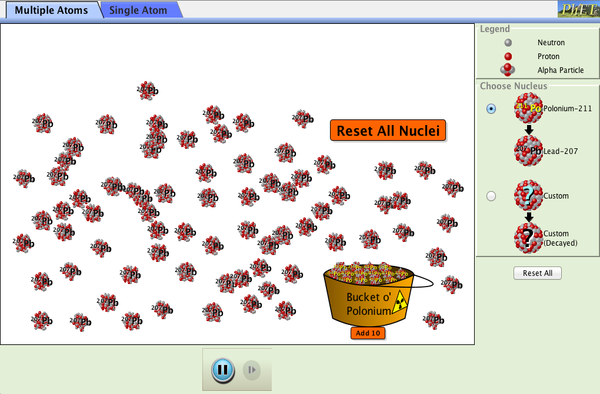
PhET
The University of Colorado has graciously allowed us to use the following PhET simulation. This simulation illustrates how radioactive nuclei decay through alpha decay, and shows the half-life of these atoms.
For further reading
For further information please see the related pages below:
References
- ↑ Wikimedia Commons. (July 22, 2015). Alpha Decay [Online]. Available: https://upload.wikimedia.org/wikipedia/commons/thumb/7/79/Alpha_Decay.svg/1280px-Alpha_Decay.svg.png
- ↑ Jefferson Lab. (July 22, 2015). Alpha Decay [Online]. Available: http://education.jlab.org/glossary/alphadecay.html
- ↑ 3.0 3.1 HyperPhysics. (July 22, 2015). Alpha Radioactivity [Online]. Available: http://hyperphysics.phy-astr.gsu.edu/hbase/nuclear/radact.html#c2
- ↑ StudyPhysics. (July 22, 2015). Alpha, Beta, and Gamma Decay [Online]. Available: http://www.studyphysics.ca/2007/30/08_atomic/43_decay.pdf
- ↑ Chubu Electric Power. (May 26, 2015). Characteristics of radiation and radioactivity [Online]. Available: http://hamaoka.chuden.jp/english/radioactivity/aspect.html
- ↑ Created internally by a member of the Energy Educaation team.
- ↑ US EPA. (July 22, 2015). Alpha Particles [Online]. Available: http://www.epa.gov/radiation/understand/alpha.html#properties
- ↑ By MD111 (https://www.flickr.com/photos/md111/3266158320/) [CC BY-SA 2.0 (https://creativecommons.org/licenses/by-sa/2.0)], via Wikimedia Commons (Accessed May 8th, 2018.) from https://upload.wikimedia.org/wikipedia/commons/2/23/InsideSmokeDetector.jpg
- ↑ BBC Bitesized. (July 22, 2015). Uses of Radiation [Online]. Available: http://www.bbc.co.uk/schools/gcsebitesize/science/ocr_gateway_pre_2011/living_future/4_nuclear_radiation2.shtml
- ↑ 10.0 10.1 ChemTeacher. (July 22, 2015). Alpha Decay [Online]. Available: http://chemteacher.chemeddl.org/services/chemteacher/index.php?option=com_content&view=article&id=65