Gamma decay
Gamma decay is one type of radioactive decay that a nucleus can undergo. What separates this type of decay process from alpha or beta decay is that no charged particles are ejected from the nucleus when it undergoes this type of decay. Instead, a high energy form of electromagnetic radiation - a gamma ray photon - is released. Gamma rays are photons that have extremely high energies which are highly ionizing.[1] As well, gamma radiation is unique in the sense that undergoing gamma decay does not change the structure or composition of the atom. Instead, it only changes the energy of the atom since the gamma ray carries no charge nor does it have an associated mass.
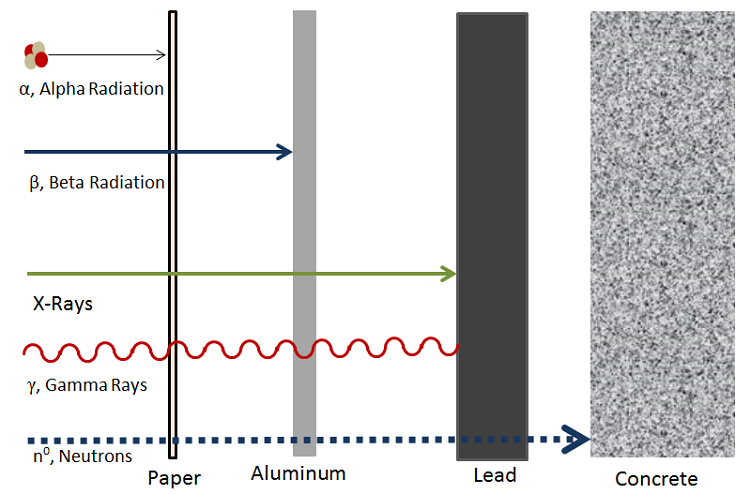
In order for a nucleus to undergo gamma decay, it must be in some sort of excited energetic state. Experiments have shown that protons and neutrons are located in discrete energy states within the nucleus, not too different from the excited states that electrons can occupy in atoms.[4] Thus if a proton or a neutron inside of the nucleus jumps up to an excited state - generally following an alpha or beta decay - the new daughter nucleus must somehow release energy to allow the proton or neutron to relax back down to ground state. When the nucleon makes this transition from a high to a low energy state, a gamma photon is emitted. The general equation that represents this process is:
where:
- is the excited atom
- is a relaxed state of the initial excited atom
- is the released gamma ray photon
Knowing that an atom undergoes gamma radiation is important, but it is also possible to determine the frequency of the released gamma radiation if the initial and final states of the nucleon inside the nucleus are known. The equation representing the frequency of the gamma radiation is:[4]
where:
- is the initial, higher energy state of the nucleon
- is the final, lower energy state of the nucleon
- is Planck's constant
- is the frequency of the emitted radiation
In addition to radioactive nuclei, there are many objects in space that emit high levels of gamma radiation.
Applications and Importance
Gamma rays can at times be harmful due to the fact that they are generally very high energy and therefore penetrate matter very easily. Since it penetrates so easily, it is some of the most useful radiation for medical purposes.[5]
I'm not quite sure about the examples given, while true in the strictest sense. Co-60 has seen far more use as a radionuclide than Cs-137 since Co-60 was used in external source devices whereas Cs-137 was only really used in LDR Brachytherapy. Conversely if memory serves me correctly Cs-137 is the more common irradiation source because of its longer half-life.
The the in the Tc part changes the meaning slightly, when present it's implying that Tc99 is the most commonly used isotope (true) whereas without the the the sentence implies that the Tc99's most common use is as a medical isotope. Since both are true it does not matter but could we display this more directly.
Some of the most widely used gamma emitters are cobalt-60, cesium-137, and technetium-99m. Cesium is used widely in radiotherapy - the treatment of cancer using gamma rays - as well as being used to measure soil density at construction sites and to investigate the subterranean layers of the Earth in oil wells. Cobalt is used to sterilize medical equipment and irradiate food, killing bacteria and pasteurizing the food. Technetium-99m (which has a shorter half-life than technetium-99) is the most widely used for diagnostic medical tests to investigate the brain, bone, and internal organs. As well, exposure to gamma radiation can improve the durability of wood and plastics, and is thus used to toughen flooring in high-traffic areas.[1]
In addition, uranium-238 and uranium-235 - used in fuel for nuclear power plants - undergo both alpha and gamma decays when used. Immediately following the fission process, gamma rays are released, resulting in high levels of radiation present around the reactor. However, safety precautions are in line to ensure that workers do not get close enough to this radioactive area to be harmed.[6]
References
- ↑ 1.0 1.1 US EPA. (May 14, 2015). Gamma Rays [Online]. Available: http://www.epa.gov/radiation/understand/gamma.html#use
- ↑ Chubu Electric Power. (May 26, 2015). Characteristics of radiation and radioactivity [Online]. Available: http://hamaoka.chuden.jp/english/radioactivity/aspect.html
- ↑ 3.0 3.1 Created internally by a member of the Energy Educaation team. Cite error: Invalid
<ref>tag; name "pic" defined multiple times with different content - ↑ 4.0 4.1 Physics Handbook. (May 14, 2015). Gamma Decay [Online]. Available: http://www.physicshandbook.com/topic/topicg/gamma.htm
- ↑ HyperPhysics. (May 14, 2015). Radioactivity [Online]. Available: http://hyperphysics.phy-astr.gsu.edu/hbase/nuclear/radact2.html#c1
- ↑ Nuclear Regulatory Commission. (May 14, 2015). Radiation Sources at Nuclear Plants [Online]. Available: http://www.nrc.gov/reading-rm/basic-ref/teachers/07.pdf