Alkene: Difference between revisions
J.williams (talk | contribs) m (1 revision imported) |
No edit summary |
||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2017-07-01]] | ||
<onlyinclude>An '''alkene''' is one of the four main types of [[hydrocarbon]]s. Alkenes are true hydrocarbons, meaning they are made up of | <onlyinclude>An '''alkene''' is one of the four main types of [[hydrocarbon]]s. Alkenes must contain at least one carbon to carbon double bond in their chain. Alkenes are true hydrocarbons, meaning they are made up of only [[hydrogen]] and [[carbon]].</onlyinclude><ref>“Alkenes,” Chemistry LibreTexts, 28-Nov-2016. [Online]. Available: http://chemwiki.ucdavis.edu/Organic_Chemistry/Hydrocarbons/Alkenes. [Accessed: 19-May-2017].</ref> | ||
Alkenes have the molecular formula C<sub>'''''n''''' </sub>H<sub>2'''''n'''''</sub>, where: | |||
*C is Carbon. | |||
* | *H is Hydrogen. | ||
* | *'''''n''''' refers to the number of carbon [[atom]]s. | ||
Alkenes are similar to [[Alkane]]s except they contain a double bond between two carbon atoms. When one carbon shares a double bond with another, this limits the number of hydrogen which can be bonded (resulting in less hydrogen atoms per carbon atom). | Alkenes are similar to [[Alkane]]s except they contain a double bond between two carbon atoms. When one carbon shares a double bond with another, this limits the number of hydrogen which can be bonded (resulting in less hydrogen atoms per carbon atom). | ||
The two simplest alkenes are [[ethene]] (C<sub>2</sub>H<sub>4</sub>) and [[propene]] (C<sub>3</sub>H<sub>6</sub>). | |||
<gallery mode = " packed" | caption="Ball and Stick Model of the Two Simplest Alkenes" |align=left> | |||
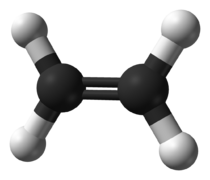
File:Ethylene-CRC-MW-3D-balls.png|300px|link=Ethene|150px|Figure 1. Eth'''ene'''.<ref>https://commons.wikimedia.org/wiki/File:Ethylene-CRC-MW-3D-balls.png</ref> | |||
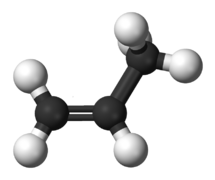
File:Propylene-3D-balls.png|link=Propene|200px|Figure 2. Prop'''ene'''.<ref>https://commons.wikimedia.org/wiki/File:Propylene-3D-balls.png</ref> | |||
</gallery> | |||
When there are 4 or more carbons in a chain, the position of the double bond can create different possible structures and uses a more precise nomenclature. Compounds that have the same chemical formula but have different bonding arrangements are called [[chemical isomer|structural isomers]] | |||
To learn more about alkenes, click [http://chemwiki.ucdavis.edu/Organic_Chemistry/Hydrocarbons/Alkenes here]. | To learn more about alkenes, click [http://chemwiki.ucdavis.edu/Organic_Chemistry/Hydrocarbons/Alkenes here]. | ||
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
[[Category:Uploaded]] | [[Category:Uploaded]] | ||
Revision as of 01:55, 29 August 2017
An alkene is one of the four main types of hydrocarbons. Alkenes must contain at least one carbon to carbon double bond in their chain. Alkenes are true hydrocarbons, meaning they are made up of only hydrogen and carbon.[1]
Alkenes have the molecular formula Cn H2n, where:
- C is Carbon.
- H is Hydrogen.
- n refers to the number of carbon atoms.
Alkenes are similar to Alkanes except they contain a double bond between two carbon atoms. When one carbon shares a double bond with another, this limits the number of hydrogen which can be bonded (resulting in less hydrogen atoms per carbon atom).
The two simplest alkenes are ethene (C2H4) and propene (C3H6).
When there are 4 or more carbons in a chain, the position of the double bond can create different possible structures and uses a more precise nomenclature. Compounds that have the same chemical formula but have different bonding arrangements are called structural isomers
To learn more about alkenes, click here.
References
- ↑ “Alkenes,” Chemistry LibreTexts, 28-Nov-2016. [Online]. Available: http://chemwiki.ucdavis.edu/Organic_Chemistry/Hydrocarbons/Alkenes. [Accessed: 19-May-2017].
- ↑ https://commons.wikimedia.org/wiki/File:Ethylene-CRC-MW-3D-balls.png
- ↑ https://commons.wikimedia.org/wiki/File:Propylene-3D-balls.png