Alkyne: Difference between revisions
J.williams (talk | contribs) m (1 revision imported) |
No edit summary |
||
| Line 1: | Line 1: | ||
[[Category: | [[Category: Amanda edit]] | ||
<onlyinclude>An '''alkyne''' is one of the four main types of [[hydrocarbon]]s. Alkynes are true hydrocarbons, meaning they are made up of nothing but [[hydrogen]] and [[carbon]].</onlyinclude><ref>http://chemwiki.ucdavis.edu/Organic_Chemistry/Hydrocarbons/Alkynes</ref> | <!--T:1--> | ||
<onlyinclude>An '''alkyne''' is one of the four main types of [[hydrocarbon]]s. Alkynes must contain at least one carbon to carbon triple bond in their chain. Alkynes are true hydrocarbons, meaning they are made up of nothing but [[hydrogen]] and [[carbon]].</onlyinclude><ref>“Alkynes,” Chemistry LibreTexts, 28-Nov-2016. [Online]. Available: http://chemwiki.ucdavis.edu/Organic_Chemistry/Hydrocarbons/Alkynes. [Accessed: 19-May-2017].</ref> | |||
<!--T:2--> | |||
* | Alkynes are based on the formula CH<sub>2'''''n'''''-2</sub>, where | ||
* | *C is Carbon. | ||
* | *H is Hydrogen. | ||
*'''''n''''' refers to the number of carbon [[atom]]s. | |||
<!--T:3--> | |||
Alkynes are similar to [[Alkane]]s except they contain a triple bond between two carbon atoms. When one carbon shares a triple bond with another, this limits the number of hydrogen which can be bonded (resulting in less hydrogen atoms per carbon atom). | Alkynes are similar to [[Alkane]]s except they contain a triple bond between two carbon atoms. When one carbon shares a triple bond with another, this limits the number of hydrogen which can be bonded (resulting in less hydrogen atoms per carbon atom). | ||
<!--T:4--> | |||
The three simplest alkynes are [[ethyne]] (C<sub>2</sub>H<sub>2</sub>), [[propyne]] (C<sub>3</sub>H<sub>4</sub>) and [[butyne]] (C<sub>4</sub>H<sub>6</sub>). | |||
<!--T:5--> | |||
<gallery mode = " packed" | caption="Ball and Stick Model of the Three Simplest Alkynes" |align=left> | |||
File:Acetylene-CRC-IR-3D-balls.png|300px|link=Ethyne|150px|Figure 1. Eth'''yne'''.<ref>https://commons.wikimedia.org/wiki/File:Acetylene-CRC-IR-3D-balls.png</ref> | |||
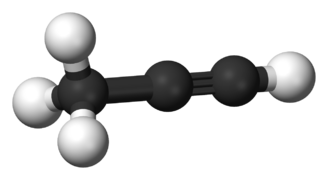
File:Propyne-3D-balls-B.png|link=Propyne|200px|Figure 2. Prop'''yne'''.<ref>https://commons.wikimedia.org/wiki/File:Propyne-3D-balls-B.png</ref> | |||
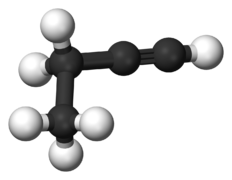
File:1-butyne-3D-balls-A.png|link=Butyne|250px|Figure 3. But'''yne'''.<ref>https://commons.wikimedia.org/wiki/File:1-butyne-3D-balls-A.png</ref> | |||
<!--T:6--> | |||
</gallery> | |||
<!--T:7--> | |||
The suffix -'''yne''' distinguishes alkynes from other hydrocarbons. | |||
To learn more about alkynes, click [http://chemwiki.ucdavis.edu/Organic_Chemistry/Hydrocarbons/Alkynes here]. | To learn more about alkynes, click [http://chemwiki.ucdavis.edu/Organic_Chemistry/Hydrocarbons/Alkynes here]. | ||
==References== | ==References== <!--T:8--> | ||
{{reflist}} | {{reflist}} | ||
[[Category:Uploaded]] | [[Category:Uploaded]] | ||
Revision as of 01:56, 29 August 2017
An alkyne is one of the four main types of hydrocarbons. Alkynes must contain at least one carbon to carbon triple bond in their chain. Alkynes are true hydrocarbons, meaning they are made up of nothing but hydrogen and carbon.[1]
Alkynes are based on the formula CH2n-2, where
- C is Carbon.
- H is Hydrogen.
- n refers to the number of carbon atoms.
Alkynes are similar to Alkanes except they contain a triple bond between two carbon atoms. When one carbon shares a triple bond with another, this limits the number of hydrogen which can be bonded (resulting in less hydrogen atoms per carbon atom).
The three simplest alkynes are ethyne (C2H2), propyne (C3H4) and butyne (C4H6).
- Ball and Stick Model of the Three Simplest Alkynes
Figure 1. Ethyne.[2]
Figure 2. Propyne.[3]
Figure 3. Butyne.[4]
The suffix -yne distinguishes alkynes from other hydrocarbons.
To learn more about alkynes, click here.
References
- ↑ “Alkynes,” Chemistry LibreTexts, 28-Nov-2016. [Online]. Available: http://chemwiki.ucdavis.edu/Organic_Chemistry/Hydrocarbons/Alkynes. [Accessed: 19-May-2017].
- ↑ https://commons.wikimedia.org/wiki/File:Acetylene-CRC-IR-3D-balls.png
- ↑ https://commons.wikimedia.org/wiki/File:Propyne-3D-balls-B.png
- ↑ https://commons.wikimedia.org/wiki/File:1-butyne-3D-balls-A.png