Base (chemistry)
A base or alkaline is defined as any substance with a pH greater than 7. This means that the ion concentration of hydrogen (H+) is less than the concentration of hydroxide (OH-). A higher pH means a higher basicity, and thus a higher concentration of OH- ions in the solution. These types of substances exhibit distinct properties: they feel slippery and have a bitter taste when ingested.[1]
When mixed with an acid, the pH of the new solution will balance out towards the neutral pH of 7, depending on the strength of the acid and base. This is known as neutralization.[1]
Uses
Bases have many uses, alongside their neutralizing abilities for acidic substances, such as when acid rain is neutralized by stones and minerals with a high pH. Some uses include:
- Scrubbers used in power plants make use of certain bases like Calcium hydroxide in order to chemically clean the sulfur oxides (a type of pollutant) produced by the plant.
- Antacids containing magnesium hydroxide are ingested in order to balance out acidity in the stomach in order to cure indigestion.
- Sodium hydroxide is used to make soaps, while calcium hydroxide is used to make bleaching powder.
- Sodium hydrogen carbonate (baking soda) has many applications.
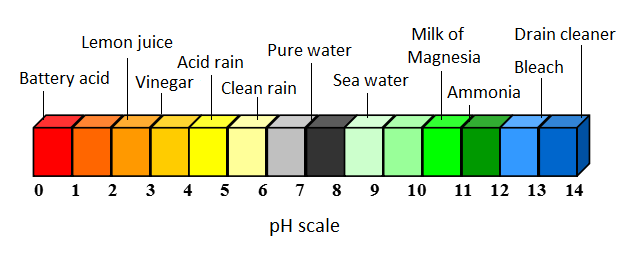
To learn more about acids and bases please see UC Davis's chem wiki.
References
- ↑ 1.0 1.1 UC Davis Chem Wiki. (July 8, 2015). Acid/Base Basics [Online], Available: http://chemwiki.ucdavis.edu/Physical_Chemistry/Acids_and_Bases/Acid#Neutralization
- ↑ Adapted from Energy: Its use and the Environment -- R. A. Hinrichs and M. Kleinbach, "Acid Rain," in Energy: Its Use and the Environment, 5th ed. Toronto, Ont. Canada: Brooks/Cole, 2006, ch.8, sec.C, pp.252-256


