Electric dipole
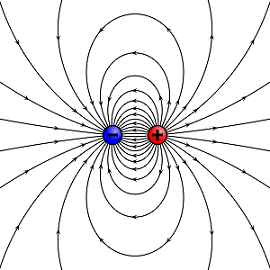
Figure 1. A dipole showing electric field lines from a positive and negative charge.[1]
An electric dipole is the separation of opposite sign charges (usually by a very small distance), typically introduced by a simple case of two charges, both with equal magnitude but opposite charge.
Electric dipoles are common in nature, so the analysis of them has many practical applications.[2] Dipoles are usually found in molecular structures caused by non-uniform charge distribution of protons and electrons, and are used to find the polarity of a system which is useful in understanding many chemical phenomena such as the normal force (the reason we don't fall through objects), surface tension, solubility, and melting/boiling points.
For more information about dipoles, please visit HyperPhysics.
For Further Reading
- Proton
- Electron
- Electric field
- Magnetic field
- Melting point & Boiling point
- System and surrounding
- Or explore a random page
References
- ↑ [GFDL (http://www.gnu.org/copyleft/fdl.html) or CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons
- ↑ R. Chabay and B. Sherwood, "The Electric Field of a Dipole," in Matter & Interactions, 3rd ed., Hoboken, NJ: Wiley, 2011, ch.14, sec.6, pp. 564-573

