Refrigerator
A refrigerator is an open system that dispels heat from a closed space to a warmer area, usually a kitchen or another room. By dispelling the heat from this area, it decreases in temperature, allowing food and other items to remain at a cool temperature. Refrigerators appear to violate the Second Law of Thermodynamics, but the key reason they do not is because of the work needed as input to the system. They are essentially heat pumps, but work to cool a region instead of heat it.[2]
How they work
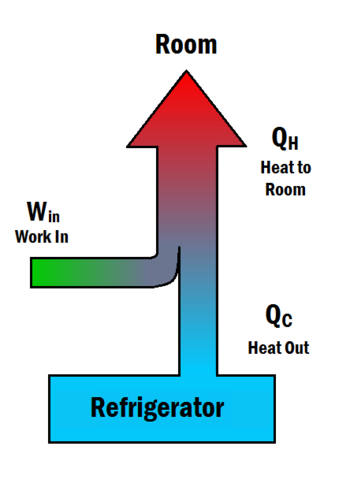
According to the Second Law of Thermodynamics, heat will always flow spontaneously from hot to cold, and never the other way around. A refrigerator causes heat to flow from cold to hot by inputting work, which cools the space inside the refrigerator. It does this by following the steps below, which can be somewhat visualized with help from Figure 1:[3]
- Work is inputted () which compresses a coolant, increasing its temperature above the room's temperature.
- Heat flows from this coolant to the air in the room (), reducing the temperature of the coolant.
- The coolant expands, and it cools down below the temperature inside the refrigerator.
- Heat flows from the refrigerator to the coolant (), decreasing the temperature inside.
This process is cyclical, and allows refrigerators to be run for as long as necessary. The work needed as input to the system is given by the equation
with the variables being shown in Figure 1. This equation shows that a refrigerator must exhaust more heat to the room than it removes from the inside. This has significant implications for whether or not you can cool a room by leaving the refrigerator door open.[2]
Efficiency
Refrigerator efficiency has improved dramatically over the years. Today U.S. refrigerators consume less than 500 kWh/year, far less than the typical 1800 kWh in 1972. Improvements were made and continue to be made in the insulation, compressor efficiency, heat exchange in the evaporator and condenser, fans, and other components of the refrigerator.[4]
U.S. Energy Star certified refrigerators must use 20% less electricity than the U.S. minimum standard for refrigerators. There is a calculator (which can be found here) that allows you to calculate yearly savings from an Energy star certified fridge, compared to the model you own, based on what you pay for electricity.[5]
Coefficient of performance (Efficiency)
For refrigerators, a manufacturer would want to make the area colder while doing as little work as possible. By doing little work to cool the appliance, the refrigerator can stay at the desired temperature while using less electricity, therefore, saving the owner money. The number that describes this idea is the coefficient of performance, , which is essentially a measure of efficiency. The equation for it is[2]
The higher this value the better, because it means that less work is being done to cool down the refrigerator.
For Further Reading
References
- ↑ Created internally by a member of the Energy Education team.
- ↑ 2.0 2.1 2.2 R. "Ideal Gas Refrigerators" in Physics for Scientists and Engineers: A Strategic Approach, 3nd ed. San Francisco, U.S.A.: Pearson Addison-Wesley, 2008, ch.19, sec.4, pp. 532 and 538
- ↑ Energy Quest (n.d.). How Does a Refrigerator Work? [Online] Available: http://www.energyquest.ca.gov/how_it_works/refrigerator.html
- ↑ Appliance Standards Awareness Project, Refrigerators and Freezers [Online], Available: http://www.appliance-standards.org/product/refrigerators-and-freezers
- ↑ Energy Star (n.d.). Refrigerator Retirement Savings Calculator [Online] Available: http://www.energystar.gov/index.cfm?fuseaction=refrig.calculator

