Efficiency
A word can have multiple and ambiguous meanings in everyday language but they have precise meanings in science. Efficiency in physics (and often for chemistry) is a comparison of the energy output to the energy input in a given system. It is defined as the percentage ratio of the output energy to the input energy, given by the equation:
This equation is commonly used in order to represent energy in the form of heat or power.
"Efficiency" is often confused with "effectiveness", and the two should be recognized as distinct from one another when analyzing energy systems. Energy efficiency measures how much a system is getting out of the fuel or primary energy flow it is using. If the energy system is effective, it is making use of this energy towards the right goal. For example, a car is a very effective form of transportation, since it is able to move people across long distances and to specific places. However, a car may not transport people very efficiently because of how it uses fuel.[2]
Types of Efficiency
Thermal Efficiency
Efficiency is very often used in science to describe how efficient a heat engine is, and is referred to as thermal efficiency.[3] This efficiency describes how much work an engine can get out of the fuel it is using. There are upper limits placed on how thermally efficient engines can be, due to the second law of thermodynamics, known as the Carnot efficiency. This Carnot efficiency only depends on the temperature of the heat source and cold sink, and is for an ideal (impossible) engine that has no change in entropy. Although such an engine would maximize efficiency, in terms of effectiveness it is terribly impractical since its idealized processes take so much time to output a significant amount of work. As Schroeder puts it, "don’t bother installing a Carnot engine in your car; while it would increase your gas mileage, you’d be passed by pedestrians".[4][5]
Electricity Transmission Efficiency
Electricity tends to lose energy in the electrical grid as it is transmitted from one location to another, depending on the magnitude of electric current, the specific conductors, and the length of the transmission line. As voltages increase these losses are reduced considerably due to its relationship with current. Typical losses from a power plant to a user in their home ranges from 8% to 15%.[6]
Wind Turbine Efficiency
Wind turbines are limited to a maximum theoretical efficiency of 59.3%, which is known as the Betz limit.[7] This law is derived by a conservation analysis of mass and momentum in the fluid flow around a wind turbine actuator. The efficiency of a wind turbine refers to how much of the energy it can get out of the wind blowing through the rotors.
Implications
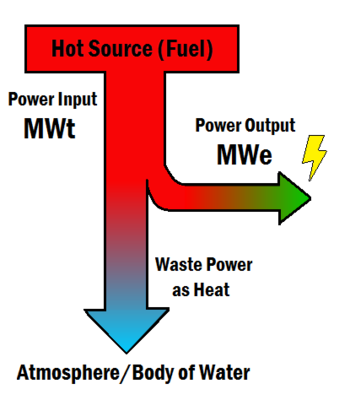
Efficiency is used to describe the energy that a certain system can extract and make useful from its energy source. Such systems include power plants, engines, and turbines. Any system that uses energy from a fuel or primary flow has a certain efficiency associated with it.
The efficiency of coal and natural gas power plants range between 32% to 42%.[8] If a power plant has an efficiency of 35% then for every 100 J of heat from coal, about 35 J becomes electricity and the other 65 J becomes heat. This heat goes into making the atmosphere warmer, or perhaps a body of water like a river or lake.
This isn't a failure of engineering, but rather a limit imposed by thermodynamics, with the maximum efficiency of such plants given by the Carnot efficiency. The lower the efficiency of such plants, the more detrimental the effects of it are on the environment as more of these fuels need to be used in order to fulfill energy needs. The ability to increase efficiency is a topic with ongoing research, primarily due to the fact that the ability to increase efficiency will lessen environmental impacts of energy use and reduce resource needs in the future. Along with efficiency, it is important for both the environment and for people's health that the right fuels are accessible.
Cogeneration plants make use of waste heat in power plants and other heat systems (like a car's engine running a heater) in order to power other parts of the system, thereby making the total efficiency higher.[9]
For Further Reading
- Second law of thermodynamics
- Conservation of energy
- Energy transformations
- Cogeneration
- Carnot efficiency
- Or explore a random page
References
- ↑ Made internally by a member of the Encyclopedia team
- ↑ Diffen, Effectiveness vs Efficiency [Online], Available: http://www.diffen.com/difference/Effectiveness_vs_Efficiency
- ↑ R. Wolfson, "Entropy, Heat Engines, and the Second Law of Thermodynamics" in Energy, Environment, and Climate, 2nd ed., New York, NY: W.W. Norton & Company, 2012, ch. 4, sec. 7, pp. 81-84
- ↑ Hyperphysics, Carnot Cycle [Online], Available: http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/carnot.html
- ↑ McMaster Physics and Astronomy, Carnot Cycle [Online], Available: http://www.physics.mcmaster.ca/~morozov/3K03/Lecture9.pdf
- ↑ IEC, EFFICIENT ELECTRICAL ENERGY TRANSMISSION AND DISTRIBUTION [Online], Available: http://www.iec.ch/about/brochures/pdf/technology/transmission.pdf
- ↑ WindPower program, The Betz limit [Online], Available: http://www.wind-power-program.com/betz.htm
- ↑ Bright Hub Engineering, The Efficiency of Power Plants of Different types [Online], Available: http://www.brighthubengineering.com/power-plants/72369-compare-the-efficiency-of-different-power-plants/
- ↑ Forbes, The Most Efficient Power Plants [Online], Available: http://www.forbes.com/2008/07/03/energy-efficiency-cogeneration-biz-energy_cx_jz_0707efficiency_horror.html