Heat: Difference between revisions
m (1 revision imported) |
energy>Ethan.boechler No edit summary |
||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2021-01-31]] | ||
[[Category: Translated to Spanish]] | |||
[[es:Calor]] | |||
[[Category:Translated to French]] | |||
[[fr:Chaleur]] | |||
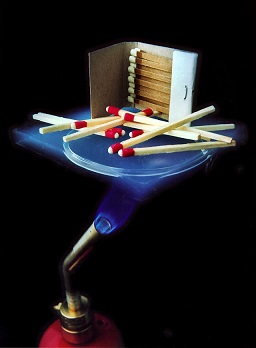
[[File:Aerogel.jpg|framed|right|Figure 1. Aerogel is a type of insulating material, meaning that it is very resistant to the flow of heat by conduction.<ref>Wikimedia Commons. (July 30, 2015). ''Aerogel'' [Online]. Available: https://upload.wikimedia.org/wikipedia/commons/b/b4/Aerogel_matches.jpg</ref>]] | [[File:Aerogel.jpg|framed|right|Figure 1. Aerogel is a type of insulating material, meaning that it is very resistant to the flow of heat by conduction.<ref>Wikimedia Commons. (July 30, 2015). ''Aerogel'' [Online]. Available: https://upload.wikimedia.org/wikipedia/commons/b/b4/Aerogel_matches.jpg</ref>]] | ||
<onlyinclude>'''Heat''' is a transfer of [[thermal energy]] caused by a difference in [[temperature]]. This temperature difference is also called a [[temperature gradient]].<ref>R. Chabay and B. Sherwood, "Fundamental Limitations on Efficiency," in Matter & Interactions, 3rd ed., Hoboken, NJ: Wiley, 2011, ch.13, sec.7, pp. 534</ref></onlyinclude> Since heat is a movement of [[energy]], it is measured in the same [[units]] as energy: [[joule]]s (J). It should also be noted that [[work]] and heat are closely related (see [[heat vs work]] for more information). | <onlyinclude>'''Heat''' is a transfer of [[thermal energy]] caused by a difference in [[temperature]]. This temperature difference is also called a [[temperature gradient]].<ref>R. Chabay and B. Sherwood, "Fundamental Limitations on Efficiency," in Matter & Interactions, 3rd ed., Hoboken, NJ: Wiley, 2011, ch.13, sec.7, pp. 534</ref></onlyinclude> Since heat is a movement of [[energy]], it is measured in the same [[units]] as energy: [[joule]]s (J). It should also be noted that [[work]] and heat are closely related (see [[heat vs work]] for more information). Both can change the temperature of a substance, and heat can be turned into work (not perfectly) and work can be turned into heat. This equivalence is the basis of how [[heat engine]]s power [[High energy society|modern society]]. | ||
'''Note''': | '''Note''': When taking courses on energy, it's important to understand that heat and temperature are '''not''' the same. Confusion about the difference between heat and temperature often leads to getting the wrong answer on exams. For an explanation on why, visit [[heat vs temperature]]. | ||
The [[second law of thermodynamics]] | The [[second law of thermodynamics]] explains why heat will always flow spontaneously from higher temperatures to lower temperatures. This energy flow can be harnessed by a [[heat engine]] to do useful work. [[Heat pump]]s can also force thermal energy to flow backward (from cold to hot), but these require energy input. | ||
==Methods of heat transfer== | ==Methods of heat transfer== | ||
| Line 12: | Line 15: | ||
There are three [[heat transfer mechanisms]]:<ref>Hyperphysics, ''Heat Transfer'' [Online], Available: http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/heatra.html</ref> | There are three [[heat transfer mechanisms]]:<ref>Hyperphysics, ''Heat Transfer'' [Online], Available: http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/heatra.html</ref> | ||
*'''[[Conduction]]''' occurs between objects that are touching each other. Collisions between small particles allow fast moving | *'''[[Conduction]]''' occurs between objects that are touching each other. Collisions between small particles allow fast-moving or vibrating particles to give some of their microscopic [[kinetic energy]] to slower particles. | ||
*'''[[Convection]]''' is [[heat transfer]] caused by moving [[fluid]]s. In a fluid, particles can mix together, move faster, and spread out, thus distributing their thermal energy. Warm [[air]] coming from a [[heating registers and vents|heating vent]] to flow around a cool room is an example of convection. | *'''[[Convection]]''' is [[heat transfer]] caused by moving [[fluid]]s. In a fluid, particles can mix together, move faster, and spread out, thus distributing their thermal energy. Warm [[air]] coming from a [[heating registers and vents|heating vent]] to flow around a cool room is an example of convection. | ||
*'''[[Radiation]]''' occurs without the movement of [[matter]]. Thermal radiation is made of electromagnetic waves given off by moving particles.<ref>R. Chabay and B. Sherwood, "Energy and Momentum in Radiation," in Matter & Interactions, 3rd ed., Hoboken, NJ: Wiley, 2011, ch.24, sec.5, pp. 1002-1003</ref> These waves can also be absorbed by materials. Microwave ovens work by radiation and the entire surface of the Earth is heated by the [[sun]]'s [[solar radiation]]. | *'''[[Radiation]]''' occurs without the movement of [[matter]]. Thermal radiation is made of electromagnetic waves given off by moving particles.<ref>R. Chabay and B. Sherwood, "Energy and Momentum in Radiation," in Matter & Interactions, 3rd ed., Hoboken, NJ: Wiley, 2011, ch.24, sec.5, pp. 1002-1003</ref> These waves can also be absorbed by materials. Microwave ovens work by radiation and the entire surface of the Earth is heated by the [[sun]]'s [[solar radiation]]. | ||
See [[heat transfer mechanisms]] for general information on how heat moves. | See [[heat transfer mechanisms]] for general information on how heat moves. | ||
| Line 22: | Line 26: | ||
==For Further Reading== | ==For Further Reading== | ||
*[[Heat vs temperature]] | *[[Heat vs temperature]] | ||
*[[Heat vs work]] | |||
*[[Thermal equilibrium]] | *[[Thermal equilibrium]] | ||
*[[Second law of thermodynamics]] | *[[Second law of thermodynamics]] | ||
Revision as of 18:56, 18 June 2021
Heat is a transfer of thermal energy caused by a difference in temperature. This temperature difference is also called a temperature gradient.[2] Since heat is a movement of energy, it is measured in the same units as energy: joules (J). It should also be noted that work and heat are closely related (see heat vs work for more information). Both can change the temperature of a substance, and heat can be turned into work (not perfectly) and work can be turned into heat. This equivalence is the basis of how heat engines power modern society.
Note: When taking courses on energy, it's important to understand that heat and temperature are not the same. Confusion about the difference between heat and temperature often leads to getting the wrong answer on exams. For an explanation on why, visit heat vs temperature.
The second law of thermodynamics explains why heat will always flow spontaneously from higher temperatures to lower temperatures. This energy flow can be harnessed by a heat engine to do useful work. Heat pumps can also force thermal energy to flow backward (from cold to hot), but these require energy input.
Methods of heat transfer
There are three heat transfer mechanisms:[3]
- Conduction occurs between objects that are touching each other. Collisions between small particles allow fast-moving or vibrating particles to give some of their microscopic kinetic energy to slower particles.
- Convection is heat transfer caused by moving fluids. In a fluid, particles can mix together, move faster, and spread out, thus distributing their thermal energy. Warm air coming from a heating vent to flow around a cool room is an example of convection.
- Radiation occurs without the movement of matter. Thermal radiation is made of electromagnetic waves given off by moving particles.[4] These waves can also be absorbed by materials. Microwave ovens work by radiation and the entire surface of the Earth is heated by the sun's solar radiation.
See heat transfer mechanisms for general information on how heat moves.
For Further Reading
- Heat vs temperature
- Heat vs work
- Thermal equilibrium
- Second law of thermodynamics
- Conduction
- Convection
- Radiation
- Or explore a random page
References
- ↑ Wikimedia Commons. (July 30, 2015). Aerogel [Online]. Available: https://upload.wikimedia.org/wikipedia/commons/b/b4/Aerogel_matches.jpg
- ↑ R. Chabay and B. Sherwood, "Fundamental Limitations on Efficiency," in Matter & Interactions, 3rd ed., Hoboken, NJ: Wiley, 2011, ch.13, sec.7, pp. 534
- ↑ Hyperphysics, Heat Transfer [Online], Available: http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/heatra.html
- ↑ R. Chabay and B. Sherwood, "Energy and Momentum in Radiation," in Matter & Interactions, 3rd ed., Hoboken, NJ: Wiley, 2011, ch.24, sec.5, pp. 1002-1003

