Hexane: Difference between revisions
J.williams (talk | contribs) m (1 revision imported) |
No edit summary |
||
| Line 1: | Line 1: | ||
[[Category:Done 2015-06 | [[Category:Done 2015-09-06]] | ||
<onlyinclude>'''Hexane''' is a [[hydrocarbon]] that can be burned as a [[fuel]]. | <onlyinclude>'''Hexane''' is a [[hydrocarbon]] that can be burned as a [[fuel]]. Its [[chemical]] formula is C<sub>6</sub>H<sub>14</sub>, and it is a [[volatile]], colourless [[liquid]] that is highly [[flammable]] and insoluble in [[water]].</onlyinclude><ref name=usepa> United States Environmental Protection Agency. (November 18, 2013). ''Hexane'' [Online]. Available: http://www.epa.gov/ttnatw01/hlthef/hexane.html [February 16,2015]. </ref> | ||
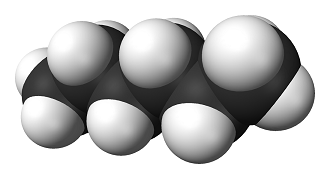
[[File:hexane.png|400px|framed|right|Figure 1. Ball and stick model of hexane, the white is [[hydrogen]] and the black is [[carbon]].<ref>"Hexane-3D-vdW.png" Licensed under Public Domain via Wikimedia Commons - http://commons.wikimedia.org/wiki/File:Hexane-3D-vdW.png</ref>]] | [[File:hexane.png|400px|framed|right|Figure 1. Ball and stick model of hexane, the white is [[hydrogen]] and the black is [[carbon]].<ref>"Hexane-3D-vdW.png" Licensed under Public Domain via Wikimedia Commons - http://commons.wikimedia.org/wiki/File:Hexane-3D-vdW.png</ref>]] | ||
| Line 20: | Line 20: | ||
| [[Molar mass]] || 86.17 [[kilogram|gram]]s/[[mole]] | | [[Molar mass]] || 86.17 [[kilogram|gram]]s/[[mole]] | ||
|- | |- | ||
| Melting | | [[Melting point]] || -95<sup>o</sup>C<ref name =elmhurst>Charles E. Ophardt. (2003). ''Virtual Chembook - Hydrocarbon Boiling Points'' [Online]. Available: http://www.elmhurst.edu/~chm/vchembook/501hcboilingpts.html [February 16,2015].</ref> | ||
|- | |- | ||
| Boiling | | [[Boiling point]] || 69<sup>o</sup>C<ref name =elmhurst/> | ||
|} | |} | ||
| Line 28: | Line 28: | ||
{{reflist}} | {{reflist}} | ||
[[Category:Uploaded]] | [[Category:Uploaded]] | ||
[[category:Ian edit]] | |||
Revision as of 19:23, 17 September 2015
Hexane is a hydrocarbon that can be burned as a fuel. Its chemical formula is C6H14, and it is a volatile, colourless liquid that is highly flammable and insoluble in water.[1]
Hexane has several important uses. Hexane is used frequently in the production and extraction of edible oils from nut and vegetable crops, such as soybeans and peanuts. As well, hexane has several industrial uses. Primarily, when used in commercial grades, hexane is used as a solvent for glues and varnishes where water cannot be used. This is because of the fact that hexane works as a solvent where water does not, due primarily to its polarity. It is also used as a cleaning agent known as a degreaser in the printing industry.[1]
Hexane also undergoes hydrocarbon combustion, combining with oxygen to form carbon dioxide. The balanced chemical equation for the complete combustion of hexane is:
Properties
Below is a table of some of the basic properties of hexane.
| Chemical formula | C6H14 |
| Molar mass | 86.17 grams/mole |
| Melting point | -95oC[3] |
| Boiling point | 69oC[3] |
References
- ↑ 1.0 1.1 United States Environmental Protection Agency. (November 18, 2013). Hexane [Online]. Available: http://www.epa.gov/ttnatw01/hlthef/hexane.html [February 16,2015].
- ↑ "Hexane-3D-vdW.png" Licensed under Public Domain via Wikimedia Commons - http://commons.wikimedia.org/wiki/File:Hexane-3D-vdW.png
- ↑ 3.0 3.1 Charles E. Ophardt. (2003). Virtual Chembook - Hydrocarbon Boiling Points [Online]. Available: http://www.elmhurst.edu/~chm/vchembook/501hcboilingpts.html [February 16,2015].