Hydrocarbon combustion
Hydrocarbon combustion refers to the chemical reaction where a hydrocarbon reacts with oxygen to create carbon dioxide, water, and heat. Hydrocarbons are molecules consisting of both hydrogen and carbon. They are most famous for being the primary constituent of fossil fuels, namely natural gas, petroleum, and coal. For this reason, fossil fuel resources are often referred to as hydrocarbon resources.[1] Energy is obtained from fossil fuels through combustion (burning) of the fuel. Although impurities exist in fossil fuels, hydrocarbon combustion is the primary process in the burning of fossil fuel. An example of hydrocarbon combustion is illustrated in Figure 1. See simulation at the bottom of the page for more examples.
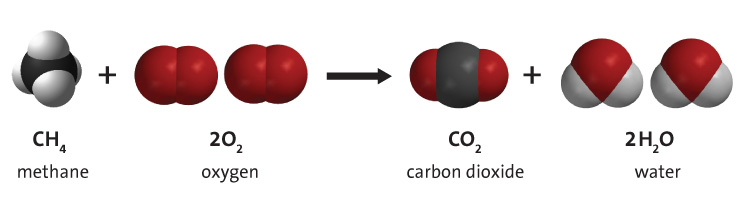
Description
Regardless of the type of hydrocarbon, combustion with oxygen produces 3 products: carbon dioxide, water and heat, as shown in the general reaction below. The energy required to break the bonds in the hydrocarbon molecules is substantially less than the energy released in the formation of the bonds in the CO2 and H2O molecules. For this reason, the process releases significant amounts of thermal energy (heat). This thermal energy can be used directly (perhaps to heat a home) or else it can be converted to mechanical energy, using a heat engine. However, this is subject to efficiency losses, resulting in necessary significant energy losses (as waste heat) governed by the second law of thermodynamics. The resulting useful mechanical energy will be a lot less than the initial thermal energy provided by the hydrocarbon combustion.
General Reaction Equation:
- refers to the number of carbon atoms in the hydrocarbon
- refers to the number of hydrogen atoms in the hydrocarbon
- refers to the number of oxygen atoms required in the hydrocarbon combustion reaction
Hydrocarbon Combustion and Fossil Fuels
Note that CO2 is always produced in hydrocarbon combustion; it doesn't matter what type of hydrocarbon molecule. Producing CO2 and H2O is actually how useful energy is obtained from fossil fuels. For this reason, it is important to distinguish between carbon dioxide and other "waste" products that arise from impurities in the fuel such as sulfur and nitrogen compounds.[1] Wastes that arise from impurities can be eliminated with the right technology; CO2 cannot be eliminated unless the fossil fuels are not burned (used) in the first place.
Not all fossil fuels have the same composition. Natural gas is composed of over 90% methane (CH4) which is the smallest hydrocarbon molecule. Oil tends to be composed of medium sized molecules, although composition varies greatly from one grade of crude to the next. In general, the denser the oil, the longer the carbon chains in the molecules. Finally coal contains the largest and most complex hydrocarbon molecules.[1]
Since different hydrocarbons have different ratios of hydrogen to carbon, they produce different ratios of water to carbon dioxide. In general, the longer and more complex the molecule, the greater the ratio of carbon to hydrogen. For this reason, combustion of equal amounts of different hydrocarbons will yield different quantities of carbon dioxide, depending on the ratio of carbon to hydrogen in molecules of each. Since coal contains the longest and most complex hydrocarbon molecules, burning coal releases more CO2 than burning the same mass of oil or natural gas. This also changes the energy density of each of these fuels.
Carbon dioxide emissions
Below is a chart of the CO2 emitted from the production of 293.1 kWh (1,000,000 BTUs) of energy from various hydrocarbon fuels.[3]
| Fuel | kg of CO2 emissions |
|---|---|
| Anthracite Coal | 104 |
| Bituminous Coal | 93.5 |
| Lignite Coal | 97.9 |
| Subbituminous Coal | 97.4 |
| Diesel | 73.2 |
| Gasoline | 71.5 |
| Propane | 63.2 |
| Natural gas | 53.2 |
Combustion Animation
Choose a fuel from the drop down menu to see the net reaction that occurs during combustion.
For Further Reading
For further information please see the related pages below:
References
- ↑ 1.0 1.1 1.2 R.D. Botts, D.M. Carson, and D.Coglon. "Petroleum in our live" in Our petroleum challenge, 8th ed. Calgary:Canadian Center for Energy Development, 2013, pp. 7-15.
- ↑ American Chemical Society. "Methane and oxygen react". Internet: http://www.middleschoolchemistry.com/multimedia/chapter6/lesson1, [October 25,2013]
- ↑ http://www.eia.gov/tools/faqs/faq.cfm?id=73&t=11

