Energy density
Energy density is the amount of energy that can be stored in a given system, substance, or region of space.[2][3] Energy density can be measured in energy per volume or per mass. The higher the energy density of a system or material, the greater the amount of energy it has stored.[4]
A material can release energy in four types of reactions. These reactions are nuclear, chemical, electrochemical and electrical.[5] When calculating the amount of energy in a system most often only useful or extractable energy is measured. In scientific equations, energy density is often denoted by U.[6]
Energy density is generally expressed in two ways, although the first is more common:
- Volumetric energy density - how much energy a system contains in comparison to its volume; typically expressed in watt-hours per liter (Wh/L) or Megajoules per liter (MJ/L).[7]
- Gravimetric energy density - how much energy a system contains in comparison to its mass; typically expressed in watt-hours per kilogram (Wh/kg), or Megajoules per kilogram (MJ/kg).[7] Gravimetric energy density can also be referred to as specific energy.
Having a high energy density does not give information on how quickly this energy can be used. This knowledge is contained in a substance's power density, which describes the rate at which its energy can be put out. Typically having a high energy density goes along with a low power density. Visit energy density vs power density for more information and examples.
Fuel energy density
Many different materials can store energy, ranging from food, to diesel, to uranium. These materials are known collectively as fuels, and all of these fuels are used as energy sources for a variety of systems. When the fuels come directly from nature (like crude oil) they are primary fuels; when the fuels have to be modified so they can be used (like gasoline) they're referred to as secondary fuels. The table below shows the energy density for a variety of common fuels.
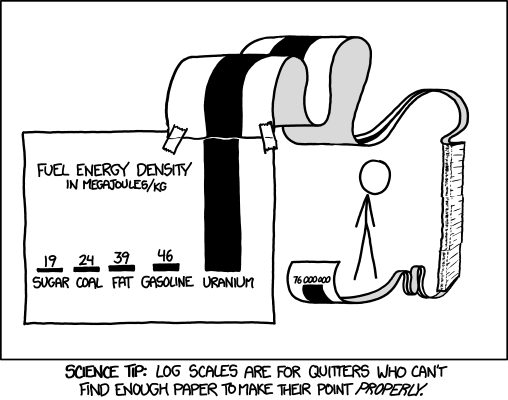
For a visual representation of these values, Figures 1 and the graph to the right show comparisons of energy densities of different fuels.
| Fuel Type | Reaction Type | Energy Density (MJ/kg) |
Typical uses |
|---|---|---|---|
| Wood | Chemical | 16 | Space heating, Cooking |
| Coal | Chemical | 24 | Power plants, Electricity generation |
| Ethanol | Chemical | 26.8 | Gasoline mixture, Alcohol, Chemical products |
| Biodiesel | Chemical | 38 [8] | automotive engine |
| Crude oil | Chemical | 44 | Refinery, Petroleum products |
| Diesel | Chemical | 45 | Diesel engines |
| Gasoline | Chemical | 46 | Gasoline engines |
| Natural gas | Chemical | 55 | Household heating, Electricity generation |
| Uranium-235 | Nuclear | 3 900 000 | Nuclear reactor electricity generation |
How far can you go?
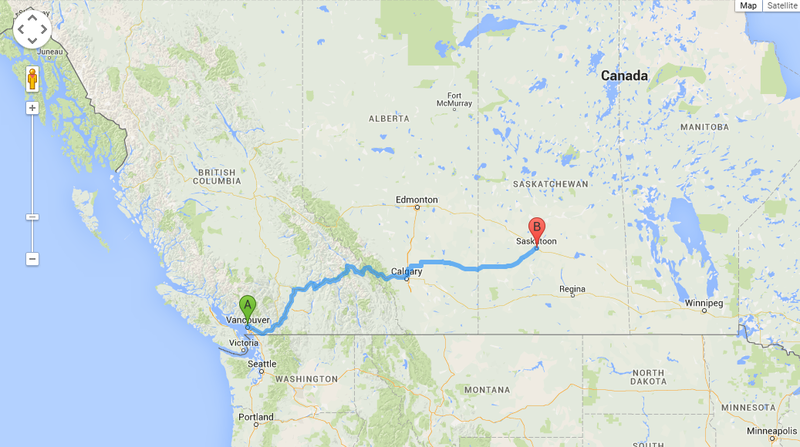
Energy sources do not give up their energy in the same ways, but assuming they could, how far would each move a vehicle? To find out, coal can be used as a base-line, if the amount of energy in a particular mass of coal equal to 10 meters - the length of a school bus. This makes the energy available in that same mass of uranium equal to the distance between Vancouver, British Columbia to Saskatoon, Saskatchewan(Figure 2). Below is a list of other fuels compared to coal to figure out the energy per distance to compare other fuels to coal.
- Wood - 7 meters, about the width of a two car garage
- Coal - 10 meters, about the length of a school bus
- Crude oil - 18 meters, roughly the length of a humpback whale
- 235Uranium - 1,625,000 m (1,625 km), greater than the distance from Vancouver to Saskatoon
To start with a different set of numbers, one kilogram of crude oil allows a car to drive ~20 km. Petroleum products, like gasoline are used because they are energy dense. A kilogram of nuclear fuel, like 235Uranium, would take a car 1.77 million km. How far is that? That's a trip from the Earth, to the moon, and back. Twice.[10] Nuclear fuels are incredibly energy-dense.
Further Reading
References
- ↑ XKCD. Log Scale [Online], Available: http://xkcd.com/1162/
- ↑ C. Dillon. (2009, October). How Far Will Energy Go? - An Energy Density Comparison [Online]. Available: http://www.cleanenergyinsight.org/interesting/how-far-will-your-energy-go-an-energy-density-comparison/
- ↑ A. Golnik and G. Elert. (2003). Energy Density of Gasoline [Online]. Available: http://hypertextbook.com/facts/2003/ArthurGolnik.shtml.
- ↑ Uni. South Carolina. (2003, October). Description of Energy and Power [Online]. Available: http://www.che.sc.edu/centers/RCS/desc_e_and_p.htm
- ↑ B. E. Layton, "A comparison of Energy Densities of Prevalent Energy Sources in Units of Joules Per Cubic Meter," Int. J. Green Energy, vol. 5, no. 6, pp. 438-455, Dec. 2008.
- ↑ E. W. Weisstein. (2007). Energy Density -- from Eric Weisstein’s World of Physics [Online]. Available: http://scienceworld.wolfram.com/physics/EnergyDensity.html
- ↑ 7.0 7.1 C. Simpson, "Characteristics of Rechargeable Batteries," National Semiconductor. Texas Instruments Inc., Dallas, 2011.
- ↑ Y. Chisti, “Biodiesel from microalgae,” Biotechnol. Adv., vol. 25, no. 3, pp. 294–306, May-Jun. 2007.
- ↑ I. Hore-Lacy, "Future Energy Demand and Supply," in Nuclear Energy in the 21st Century, 2nd ed., London, UK: WNUP, 2011, ch.1, sec.6, pp.9
- ↑ Wolfram Alpha, entry: 1772727273 m