Energy density of storage devices
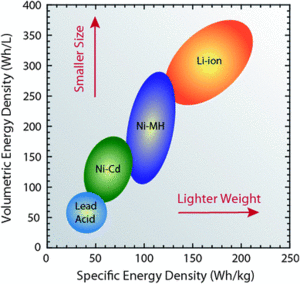
Energy density of storage devices is of great consideration when deciding which storage device to use for a given scenario. If a storage device has a larger energy density than another, this means that it can be smaller and/or weigh less while containing the same amount of energy. Considering this, small technology devices like cell phones would want a battery with a high energy density, since it must be lightweight and compact. However a car can sacrifice an increase of mass and use a lower energy density battery, since the mass of its battery is not that large compared to its other components.
An energy storage device can be used either as the energy supply for a system or as a unit to store energy from a generator. For example, a lead-acid battery can provide electrical power for a motor vehicle while a Lithium ion battery can be used to store energy for a cell phone. Compared to fuels, energy storage has the advantage of being able to recharge its energy without the need to add more materials to its system. For a visual comparison, the energy densities of the batteries are displayed in Figure 1.
It is more useful for an energy storage device to have a high energy density. This means the device will be able to supply energy over a longer period of time, which is optimal for portable electronics or vehicles.[2] However, if a storage device has a large energy density, then the power density is usually compromised.[3] (see: Energy density vs. power density)
| Energy Storage | Reaction Type | Energy Density (Wh/kg) |
Typical Uses |
|---|---|---|---|
| Lead-Acid battery | Electrochemical | 30-50 | Automobile Electronics |
| Nickel-Cadmium battery | Electrochemical | 45-80 | Portable electronics, Electric Vehicles |
| Nickel-metal hydride battery | Electrochemical | 60-120 | Portable Electronics |
| Lithium ion battery | Electrochemical | 110-200 | Computers, Mobile Devices, Electric Vehicles |
| Electric Double Layer Capacitor | Electrical | 1-150 [4] | Electronic circuits, regenerative braking |
References
- ↑ B. J. Landi et al., “Carbon nanotubes for lithium ion batteries,” Energy Environ. Sci., vol. 2, no. 6, pp. 638-654, Mar. 2009.
- ↑ J. Marcus. (2012, March, 15). Researchers develop graphene supercapacitor holding promise for portable electronics [Online]. Available: http://newsroom.ucla.edu/portal/ucla/ucla-researchers-develop-new-graphene-230478.aspx
- ↑ Uni. South Carolina. (2003, October). Description of Energy and Power [Online]. Available: http://www.che.sc.edu/centers/RCS/desc_e_and_p.htm
- ↑ F. Zhang et al., “Hybrid graphene electrodes for supercapacitors of high energy density,” Chem. Phys. Lett., vol. 584, pp. 124–129, Oct. 2013

