Isobar (pressure): Difference between revisions
Energy>Jmdonev No edit summary |
m (1 revision imported) |
||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2021-10-29]] | ||
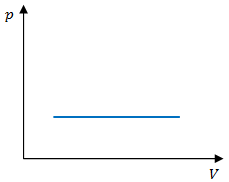
[[File:isobar-proces.png|thumb|700px|right|The [[PV diagram]] of an isobaric process<ref>Wikimedia Commons [Online], Available: http://wiki.mitsted.dk/?page=Billede:Isobar-proces.png</ref>]] | [[File:isobar-proces.png|thumb|700px|right|The [[PV diagram]] of an isobaric process<ref>Wikimedia Commons [Online], Available: http://wiki.mitsted.dk/?page=Billede:Isobar-proces.png</ref>]] | ||
<onlyinclude>An '''isobar''' in the context of [[thermodynamics]] refers to any process in which the [[system]] remains at a constant (unchanging) [[pressure]] | <onlyinclude>An '''isobar''' in the context of [[thermodynamics]] refers to any process in which the [[system]] remains at a constant (unchanging) [[pressure]]. </onlyinclude> | ||
When a system expands at | When a system expands at constant pressure, such as a [[piston]] in an [[internal combustion engine]], it's [[volume]] increases which corresponds to an output of [[work]]. However, the system cannot do this work without an input (or output) of [[heat]]. By analyzing the [[first law of thermodynamics]], the amount of heat necessary to do a given amount of work without the pressure changing can be calculated by using the system's [[specific heat capacity]]. | ||
To calculate the work done in an isobaric process, | To calculate the work done on a g as in an isobaric process, calculate the negative area under the curve. As seen in figure 1, this area will be a rectangle, so the area will be the base of the rectagle(<math>\Delta V</math>) times its height (<math>p</math>):<ref name=Knight>R. Knight, Physics for scientists and engineers. Boston, Mass.: Addison-Wesley, 2012, p. 474.</ref> | ||
<center><math>W = -p\Delta V</math></center> | <center><math>W = -p\Delta V</math></center> | ||
Therefore, a [[system and surrounding|closed system]] that is being compressed (decreasing volume), will have a positive <math>W</math> value—contrarily an expansion (increasing volume) will result in a negative <math>W</math> value | Therefore, a [[system and surrounding|closed system]] that is being compressed (decreasing volume), will have a positive <math>W</math> value—contrarily an expansion (increasing volume) will result in a negative <math>W</math> value. | ||
A real life example | <math>W > 0</math> when the gas is compressed. Energy is transferred from the environment to the gas. | ||
<math>W < 0</math> when the gas expands. Energy is transferred from the gas to the environment. | |||
For a system to compress, it requires a cooling source (or output of heat from the system) to keep the pressure constant. Otherwise, the compressed volume ''without'' the removal of heat will increase the pressure. Contrarily, for expansion the system requires an input of heat, otherwise ''without'' the addition of heat, the pressure in the container would decrease. | |||
A real-life example of an isobaric process is cooking food in an open pot. Since the pot is an [[system and surrounding|open system]], the molecules can escape (as vapour) as the contents in the pot get hotter. Referring to the ideal gas law: <math>pV = nRT</math>, if heat is added to this system temperature (<math>T</math>) increases, however, since it's an open system, molecules can escape (<math>n</math> decreases). When molecules escape this will decrease the volume (<math>V</math>). Throughout this process, the pressure stays constant. | |||
For more information, visit [http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/cppro.html#c1 Hyperphysics].[[Category:Uploaded]] | For more information, visit [http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/cppro.html#c1 Hyperphysics].[[Category:Uploaded]] | ||
| Line 21: | Line 27: | ||
*[[PV diagram]] | *[[PV diagram]] | ||
*[[Ideal gas law]] | *[[Ideal gas law]] | ||
*[[Isochore]] | *[[Isochore (volume)]] | ||
*[[Isothermal]] | *[[Isothermal]] | ||
*[[Adiabatic]] | *[[Adiabatic]] | ||
Latest revision as of 19:47, 20 December 2021
An isobar in the context of thermodynamics refers to any process in which the system remains at a constant (unchanging) pressure.
When a system expands at constant pressure, such as a piston in an internal combustion engine, it's volume increases which corresponds to an output of work. However, the system cannot do this work without an input (or output) of heat. By analyzing the first law of thermodynamics, the amount of heat necessary to do a given amount of work without the pressure changing can be calculated by using the system's specific heat capacity.
To calculate the work done on a g as in an isobaric process, calculate the negative area under the curve. As seen in figure 1, this area will be a rectangle, so the area will be the base of the rectagle() times its height ():[2]
Therefore, a closed system that is being compressed (decreasing volume), will have a positive value—contrarily an expansion (increasing volume) will result in a negative value.
when the gas is compressed. Energy is transferred from the environment to the gas.
when the gas expands. Energy is transferred from the gas to the environment.
For a system to compress, it requires a cooling source (or output of heat from the system) to keep the pressure constant. Otherwise, the compressed volume without the removal of heat will increase the pressure. Contrarily, for expansion the system requires an input of heat, otherwise without the addition of heat, the pressure in the container would decrease.
A real-life example of an isobaric process is cooking food in an open pot. Since the pot is an open system, the molecules can escape (as vapour) as the contents in the pot get hotter. Referring to the ideal gas law: , if heat is added to this system temperature () increases, however, since it's an open system, molecules can escape ( decreases). When molecules escape this will decrease the volume (). Throughout this process, the pressure stays constant.
For more information, visit Hyperphysics.
For Further Reading
- PV diagram
- Ideal gas law
- Isochore (volume)
- Isothermal
- Adiabatic
- System and surrounding
- Or explore a [random page]
References
- ↑ Wikimedia Commons [Online], Available: http://wiki.mitsted.dk/?page=Billede:Isobar-proces.png
- ↑ R. Knight, Physics for scientists and engineers. Boston, Mass.: Addison-Wesley, 2012, p. 474.