Latent heat: Difference between revisions
No edit summary |
energy>Jmdonev No edit summary |
||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2021-01-31]] | ||
<onlyinclude>'''Latent heat''' is the heat required for an object to [[phase change|change phase]] (melt, boil, freeze, etc.). This energy is closely related to [[enthalpy]]. | <onlyinclude>'''Latent heat''' is the heat required for an object to [[phase change|change phase]] (melt, boil, freeze, etc.).</onlyinclude> This energy is closely related to [[enthalpy]].<ref name=Knight>Randall Knight, ''Physics for Scientists and Engineers,'' 3rd Ed. New York: Pearson, 2013, Ch. 17, p. 482.</ref> In figure 1, very cold ice has heat added to it. The [[temperature]] goes up, so that's [[sensible heat]], but once it starts melting, that heat is latent heat (and is represented by the flat parts of the line, during melting or evaporation). | ||
[[File:Water_Phase_Change_Diagram.png|frame|Figure 1. Adding heat to water can either raise the temperature or change the phase.<Ref>Wikimedia Commons [Online], Available: https://commons.wikimedia.org/wiki/File:Water_Phase_Change_Diagram.png#/media/File:Water_Phase_Change_Diagram.png </ref> The heat that changes the temperature is sensible heat, the heat that changes the phase is latent heat.]] | [[File:Water_Phase_Change_Diagram.png|frame|center|Figure 1. Adding heat to water can either raise the temperature or change the phase.<Ref>Wikimedia Commons [Online], Available: https://commons.wikimedia.org/wiki/File:Water_Phase_Change_Diagram.png#/media/File:Water_Phase_Change_Diagram.png </ref> The heat that changes the temperature is sensible heat, the heat that changes the phase is latent heat.]] | ||
===Fusion=== | ===Fusion=== | ||
The latent heat of fusion is the heat required for an object to go from the [[solid]] state to the liquid state, or vice versa.<ref name=Knight/> Since its value is generally much higher than [[specific heat capacity|specific heat]], it allows you to keep a beverage cold for much longer by adding ice than simply having a cold liquid to begin with. It's also why frozen meat takes a long time to thaw, but once its thawed, it heats up quickly. | The latent heat of fusion is the heat required for an object to go from the [[solid]] state to the liquid state, or vice versa.<ref name=Knight/> Since its value is generally much higher than [[specific heat capacity|specific heat]], it allows you to keep a beverage cold for much longer by adding ice than simply having a cold liquid to begin with. It's also why frozen meat takes a long time to thaw, but once its thawed, it heats up quickly. The latent heat of fusion is also referred to as the [[enthalpy of fusion]]. | ||
Ice and [[water]] have enormous latent heats associated with them which is why snow takes so long to melt and boiling water is used for cooking. This is also important in keeping our planet comfortable to live on, and provides a fair amount of resistance to [[climate change]]. | Ice and [[water]] have enormous latent heats associated with them which is why snow takes so long to melt and boiling water is used for cooking. This is also important in keeping our planet comfortable to live on, and provides a fair amount of resistance to [[climate change]]. | ||
===Vaporization=== | ===Vaporization=== | ||
The latent heat of vaporization is the thermal energy required for a [[liquid]] to vaporize to a gas or the amount that is released when a gas condenses to a liquid.<ref name=Knight/> Water has a high latent heat of vaporization, which is why [[steam]] burns are so dangerous. When steam burns a person's arm for example, this energy transfer causes the steam to condense—which uses much more energy than simply changing the temperature. Hot water would also burn, however, if a person were to get a hot water burn—that would only change the temperature of the person's hand and the water, and will not cause a phase change. Therefore, steam water burns are much worse (because a phase change is actually happening). | The latent heat of vaporization is the thermal energy required for a [[liquid]] to vaporize to a gas or the amount that is released when a gas condenses to a liquid.<ref name=Knight/> The latent heat of vaporization is also referred to as the [[enthalpy of vaporization]]. Water has a high latent heat of vaporization, which is why [[steam]] burns are so dangerous. When steam burns a person's arm for example, this energy transfer causes the steam to condense—which uses much more energy than simply changing the temperature. Hot water would also burn, however, if a person were to get a hot water burn—that would only change the temperature of the person's hand and the water, and will not cause a phase change. Therefore, steam water burns are much worse (because a phase change is actually happening). | ||
==Specific Latent Heat== | |||
[[File:latent heat.png|400px|thumbnail|right|framed|Figure 2: Specific latent heat of fusion, vaporization and sublimation.<ref>"Latent heat", Cambridge University Press, 2014. [Online]. Available: https://static.cambridge.org/binary/version/id/urn:cambridge.org:id:binary:20190207075706603-0570:9781107326200:61588figu30.png?pub-status=live. [Accessed: 06-May-2021].</ref>]] | |||
The '''specific latent heat''' of a substance is the amount of energy needed to change the state of 1 kg of the substance without changing its temperature.<ref name=bbc>''Specific latent heat,'' BBC Bitesize, 2021. [Online]. Available: https://www.bbc.co.uk/bitesize/guides/zg6bdxs/revision/4. [Accessed: 06-May-2021].</ref> The distinction from '''latent heat''' is due to the term "specific," which tells us that the substance is being measured per unit [[mass]]. In addition, since '''specific latent heat''' is measured per unit mass, it is an [[intensive property]] of matter. | |||
The specific latent heat (represented by the symbol <math>L</math>) of a material is a measure of the heat energy (<math>Q</math>) per unit mass (<math>m</math>) released or absorbed during a phase change. [[Specific latent heat]] can be found using the formula <math>Q = mL</math>. Rearranging the formula we get: | |||
<math>L = \frac{Q}{m}</math> | |||
where: | |||
*<math>L</math> is the [[specific latent heat]] for a particular substance, either L<sub>f</sub> for fusion, or L<sub>v</sub> for vaporization. | |||
*<math>Q</math> is the thermal energy released or absorbed during the phase change of the substance (usually in kJ). | |||
*<math>m</math> is the mass of the substance (usually in kg). | |||
| Line 18: | Line 30: | ||
*[[Solid]], [[Liquid]], [[Gas]] | *[[Solid]], [[Liquid]], [[Gas]] | ||
*[[Steam]] | *[[Steam]] | ||
*[[Enthalpy of vaporization]], [[Enthalpy of fusion]] | |||
*[[Specific heat capacity]] | *[[Specific heat capacity]] | ||
*[[Climate change]] | *[[Climate change]] | ||
Revision as of 22:49, 25 May 2021
Latent heat is the heat required for an object to change phase (melt, boil, freeze, etc.). This energy is closely related to enthalpy.[1] In figure 1, very cold ice has heat added to it. The temperature goes up, so that's sensible heat, but once it starts melting, that heat is latent heat (and is represented by the flat parts of the line, during melting or evaporation).
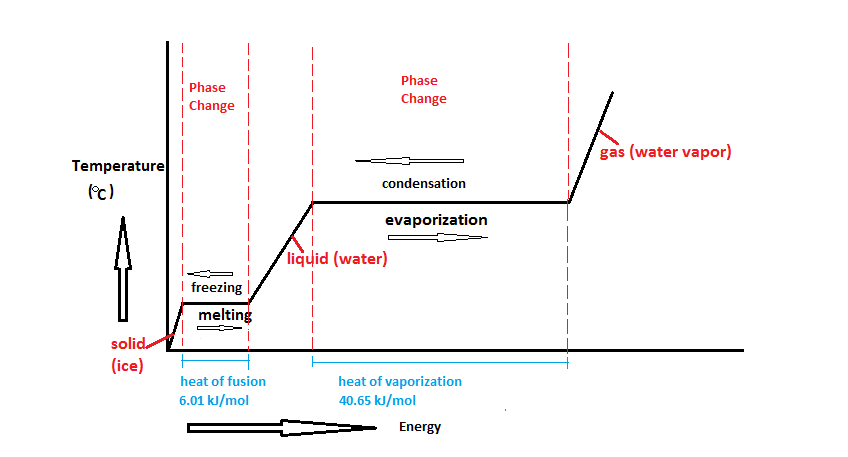
Fusion
The latent heat of fusion is the heat required for an object to go from the solid state to the liquid state, or vice versa.[1] Since its value is generally much higher than specific heat, it allows you to keep a beverage cold for much longer by adding ice than simply having a cold liquid to begin with. It's also why frozen meat takes a long time to thaw, but once its thawed, it heats up quickly. The latent heat of fusion is also referred to as the enthalpy of fusion.
Ice and water have enormous latent heats associated with them which is why snow takes so long to melt and boiling water is used for cooking. This is also important in keeping our planet comfortable to live on, and provides a fair amount of resistance to climate change.
Vaporization
The latent heat of vaporization is the thermal energy required for a liquid to vaporize to a gas or the amount that is released when a gas condenses to a liquid.[1] The latent heat of vaporization is also referred to as the enthalpy of vaporization. Water has a high latent heat of vaporization, which is why steam burns are so dangerous. When steam burns a person's arm for example, this energy transfer causes the steam to condense—which uses much more energy than simply changing the temperature. Hot water would also burn, however, if a person were to get a hot water burn—that would only change the temperature of the person's hand and the water, and will not cause a phase change. Therefore, steam water burns are much worse (because a phase change is actually happening).
Specific Latent Heat
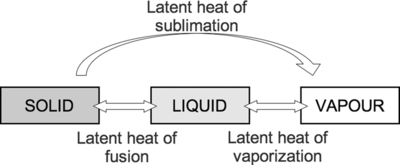
The specific latent heat of a substance is the amount of energy needed to change the state of 1 kg of the substance without changing its temperature.[4] The distinction from latent heat is due to the term "specific," which tells us that the substance is being measured per unit mass. In addition, since specific latent heat is measured per unit mass, it is an intensive property of matter.
The specific latent heat (represented by the symbol ) of a material is a measure of the heat energy () per unit mass () released or absorbed during a phase change. Specific latent heat can be found using the formula . Rearranging the formula we get:
where:
- is the specific latent heat for a particular substance, either Lf for fusion, or Lv for vaporization.
- is the thermal energy released or absorbed during the phase change of the substance (usually in kJ).
- is the mass of the substance (usually in kg).
To learn more about enthalpy and latent heat please see hyperphysics.
For Further Reading
- Solid, Liquid, Gas
- Steam
- Enthalpy of vaporization, Enthalpy of fusion
- Specific heat capacity
- Climate change
- Or explore a random page
References
- ↑ 1.0 1.1 1.2 Randall Knight, Physics for Scientists and Engineers, 3rd Ed. New York: Pearson, 2013, Ch. 17, p. 482.
- ↑ Wikimedia Commons [Online], Available: https://commons.wikimedia.org/wiki/File:Water_Phase_Change_Diagram.png#/media/File:Water_Phase_Change_Diagram.png
- ↑ "Latent heat", Cambridge University Press, 2014. [Online]. Available: https://static.cambridge.org/binary/version/id/urn:cambridge.org:id:binary:20190207075706603-0570:9781107326200:61588figu30.png?pub-status=live. [Accessed: 06-May-2021].
- ↑ Specific latent heat, BBC Bitesize, 2021. [Online]. Available: https://www.bbc.co.uk/bitesize/guides/zg6bdxs/revision/4. [Accessed: 06-May-2021].

