Sensible heat: Difference between revisions
J.williams (talk | contribs) m (1 revision imported) |
No edit summary |
||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2017-07-01]] | ||
[[File:Fire.jpg|300px|thumbnail|Figure 1: Campfires emit radiant heat which is felt as sensible heat because it increases the temperature of your body.<ref>Wikimedia Commons [Online], Available: http://commons.wikimedia.org/wiki/File:Fire_from_brazier.jpg</ref>]] | [[File:Fire.jpg|300px|thumbnail|Figure 1: Campfires emit radiant heat which is felt as sensible heat because it increases the temperature of your body.<ref>Wikimedia Commons [Online], Available: http://commons.wikimedia.org/wiki/File:Fire_from_brazier.jpg</ref>]] | ||
<onlyinclude>'''Sensible heat''' is the energy | <onlyinclude>'''Sensible heat''' is literally the heat that can be felt. It is the energy moving from one system to another that changes the [[temperature]] rather than [[phase change|changing its phase]]. For example, it warms [[water]] rather than melting [[ice]].</onlyinclude> In other words, it is the heat that can be felt standing near a fire, or standing outside on a [[sunny day. Sensible heat is used in contrast to [[latent heat]] (the heat needed to change from one form of matter to another, which doesn't change temperature), as the two are essentially opposite. | ||
For example, in a [[air conditioner|cooling system]] condensation forms due to removal of latent heat, and the refrigerant (cooling liquid) changes temperature due to sensible heat. The sensible heat capacity then describes the capacity required to lower the temperature whereas latent [[heat capacity]] is the capacity to remove the moisture from the air.<ref>Space Air, ''What is the difference between sensible and latent heat?'' [Online], Available: https://www.spaceair.co.uk/faqs/what-is-the-difference-between-sensible-and-latent-heat</ref> | For example, in a [[air conditioner|cooling system]] condensation forms due to removal of latent heat, and the refrigerant (cooling liquid) changes temperature due to sensible heat. The sensible heat capacity then describes the capacity required to lower the temperature whereas latent [[heat capacity]] is the capacity to remove the moisture from the air.<ref>Space Air, ''What is the difference between sensible and latent heat?'' [Online], Available: https://www.spaceair.co.uk/faqs/what-is-the-difference-between-sensible-and-latent-heat</ref> | ||
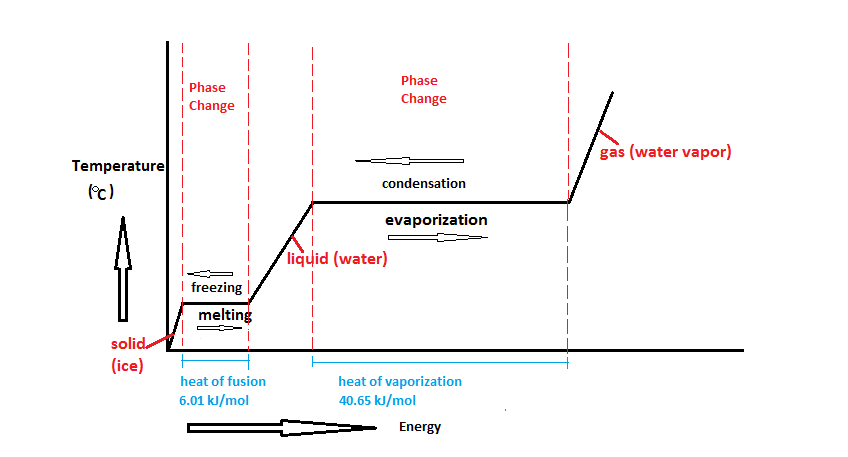
In figure 2, very cold ice has heat added to it. The [[temperature]] goes up, so that's sensible heat, but once it starts melting, that heat is latent heat (and is represented by the flat parts of the line, during melting or evaporation). | |||
[[File:Water_Phase_Change_Diagram.png|frame|Figure 2. Adding heat to water can either raise the temperature or change the phase.<Ref>Wikimedia Commons [Online], Available: https://commons.wikimedia.org/wiki/File:Water_Phase_Change_Diagram.png#/media/File:Water_Phase_Change_Diagram.png </ref> The heat that changes the temperature is sensible heat, the heat that changes the phase is latent heat.]] | |||
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
[[Category:Uploaded]] | [[Category:Uploaded]] | ||
Revision as of 22:16, 15 August 2017

Sensible heat is literally the heat that can be felt. It is the energy moving from one system to another that changes the temperature rather than changing its phase. For example, it warms water rather than melting ice. In other words, it is the heat that can be felt standing near a fire, or standing outside on a [[sunny day. Sensible heat is used in contrast to latent heat (the heat needed to change from one form of matter to another, which doesn't change temperature), as the two are essentially opposite.
For example, in a cooling system condensation forms due to removal of latent heat, and the refrigerant (cooling liquid) changes temperature due to sensible heat. The sensible heat capacity then describes the capacity required to lower the temperature whereas latent heat capacity is the capacity to remove the moisture from the air.[2]
In figure 2, very cold ice has heat added to it. The temperature goes up, so that's sensible heat, but once it starts melting, that heat is latent heat (and is represented by the flat parts of the line, during melting or evaporation).
References
- ↑ Wikimedia Commons [Online], Available: http://commons.wikimedia.org/wiki/File:Fire_from_brazier.jpg
- ↑ Space Air, What is the difference between sensible and latent heat? [Online], Available: https://www.spaceair.co.uk/faqs/what-is-the-difference-between-sensible-and-latent-heat
- ↑ Wikimedia Commons [Online], Available: https://commons.wikimedia.org/wiki/File:Water_Phase_Change_Diagram.png#/media/File:Water_Phase_Change_Diagram.png

