Separative work unit: Difference between revisions
m (1 revision imported: From the summer) |
No edit summary |
||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2018-05-18]] | ||
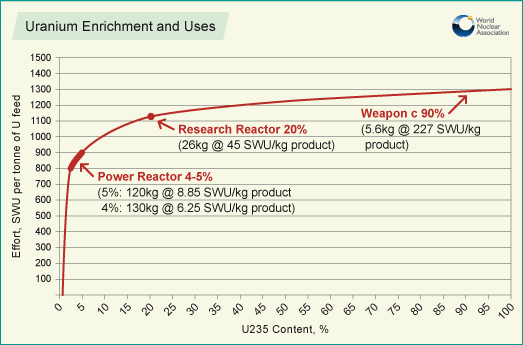
[[File:SWU.png|400px|framed|right|Figure 1. Graph showing increase in SWU with increase in <sup>235</sup>U concentration. Power reactors here depicts Light Water reactors (e.g. [[PWR]] and [[BWR]]'s).<ref>World Nuclear Association. (June 28, 2016). ''Uranium Enrichment'' [Online]. Available: http://www.world-nuclear.org/information-library/nuclear-fuel-cycle/conversion-enrichment-and-fabrication/uranium-enrichment.aspx</ref>]] | [[File:SWU.png|400px|framed|right|Figure 1. Graph showing increase in SWU with increase in <sup>235</sup>U concentration. Power reactors here depicts Light Water reactors (e.g. [[PWR]] and [[BWR]]'s).<ref>World Nuclear Association. (June 28, 2016). ''Uranium Enrichment'' [Online]. Available: http://www.world-nuclear.org/information-library/nuclear-fuel-cycle/conversion-enrichment-and-fabrication/uranium-enrichment.aspx</ref>]] | ||
<onlyinclude>The '''Separative Work Unit''' (SWU) is a unit that defines the effort required in the [[uranium enrichment]] process, in which uranium-235 and -238 are separated.</onlyinclude> The separative work unit is measured in units of [[kilogram|kg]] (kilograms) and can then be manipulated to determine [[cost]] per SWU and [[kWh]] (kilowatt hour) per SWU (it is not [[work]] in the traditional physics sense). | <onlyinclude>The '''Separative Work Unit''' (SWU) is a unit that defines the effort required in the [[uranium enrichment]] process, in which uranium-235 and -238 are separated.</onlyinclude> The separative work unit is measured in units of [[kilogram|kg]] (kilograms) and can then be manipulated to determine [[cost]] per SWU and [[kWh]] (kilowatt hour) per SWU (it is not [[work]] in the traditional physics sense). | ||
Natural [[uranium]] is 0.711% <sup>235</sup>U and needs to be enriched to between 3 and 5% for use in a light water reactor (a [[BWR]] or a [[PWR]]). In order to do this, the uranium undergoes an enrichment process that requires an input of separative work. This is due to the [[second law of thermodynamics]] which states that the [[entropy]] (disorder) of any isolated system must increase; because separated [[isotope]]s represent more order (lower entropy) than mixed isotopes, effort must be put in to enrich the uranium.<ref name="r1">John R. Lamarsh, Anthony J. Baratta. (June 28, 2016). ''Introduction to Nuclear Engineering''. Third Edition. Upper Saddle River, NJ, U.S.A:Prentice Hall, 2001.</ref> | Natural [[uranium]] is 0.711% <sup>235</sup>U and needs to be enriched to between 3 and 5% for use in a light water reactor (a [[BWR]] or a [[PWR]]). In order to do this, the uranium undergoes an enrichment process that requires an input of separative work. This is due to the [[second law of thermodynamics]] which states that the [[entropy]] (disorder) of any isolated system must increase; but because separated [[isotope]]s represent more order (lower entropy) than mixed isotopes, effort must be put in to enrich the uranium.<ref name="r1">John R. Lamarsh, Anthony J. Baratta. (June 28, 2016). ''Introduction to Nuclear Engineering''. Third Edition. Upper Saddle River, NJ, U.S.A:Prentice Hall, 2001.</ref> | ||
==Calculating SWU== | ==Calculating SWU== | ||
The amount of uranium required to be fed into the enrichment plant in order to obtain a desired amount of enriched product depends on the desired enrichment of the product, the original enrichment of the feed and the enrichment of the depleted uranium or the 'tails'. Knowing this, a [[mass]] balance equation using these three masses and their respective percent concentrations can be written:<ref name="r1"/> | The amount of uranium required to be fed into the enrichment plant in order to obtain a desired amount of enriched product depends on the desired enrichment of the product, the original enrichment of the feed and the enrichment of the depleted uranium or the 'tails'. Knowing this, a [[mass]] balance equation using these three masses and their respective percent concentrations can be written:<ref name="r1"/> | ||
<center>< | <center><math>x_{F}M_{F} = x_{P}M_{P} - x_{T}M_{T}</math>............. (Equation 1)</center> | ||
Where '''x<sub>F</sub>''' is the concentration of the feed uranium and '''M<sub>F</sub>''' is the mass of the feed uranium, '''x<sub>P</sub>''' is the concentration of the product uranium and '''M<sub>P</sub>''' is the mass of the product uranium, '''x<sub>T</sub>''' is the concentration of the tails and '''M<sub>T</sub>''' is the mass of the tails. | Where '''x<sub>F</sub>''' is the concentration of the feed uranium and '''M<sub>F</sub>''' is the mass of the feed uranium, '''x<sub>P</sub>''' is the concentration of the product uranium and '''M<sub>P</sub>''' is the mass of the product uranium, '''x<sub>T</sub>''' is the concentration of the tails and '''M<sub>T</sub>''' is the mass of the tails. | ||
| Line 15: | Line 15: | ||
Applying conservation of mass (M<sub>F</sub> = M<sub>P</sub> + M<sub>T</sub>) and manipulating the equation a little bit we're left with:<ref name="r1"/> | Applying conservation of mass (M<sub>F</sub> = M<sub>P</sub> + M<sub>T</sub>) and manipulating the equation a little bit we're left with:<ref name="r1"/> | ||
<center>< | <center><math>\frac{M_{F}}{M_{P}} = \frac{x_{P}-x_{T}}{x_{F}-x_{T}}</math>............. (Equation 2)</center> | ||
The concentration of the feed uranium will almost always be 0.711%, which is the concentration of natural uranium, therefore as long as the desired concentration of product and tails along with desired mass of product are known, mass of feed can be determined. | The concentration of the feed uranium will almost always be 0.711%, which is the concentration of natural uranium, therefore as long as the desired concentration of product and tails along with desired mass of product are known, mass of feed can be determined. | ||
| Line 21: | Line 21: | ||
Separative work can be expressed in terms of a function V(x) known as the ''value function'', given by:<ref name="r1"/> | Separative work can be expressed in terms of a function V(x) known as the ''value function'', given by:<ref name="r1"/> | ||
<center>< | <center><math>V(x) = (1-2x)\ln\left(\frac{1-x}{x}\right)</math>............. (Equation 3)</center> | ||
Where x is the enrichment concentration. | Where x is the enrichment concentration. | ||
| Line 27: | Line 27: | ||
The SWU can then be determined using the masses of each uranium concentration found using equation 2 and the value function found for each respective concentration:<ref name="r1"/> | The SWU can then be determined using the masses of each uranium concentration found using equation 2 and the value function found for each respective concentration:<ref name="r1"/> | ||
<center>< | <center><math>SWU = M_{P}V(x_{P}) + M_{T}V(x_{T}) - M_{F}V(x_{F})</math>............. (Equation 4)</center> | ||
| Line 39: | Line 39: | ||
Here we use Equation 2: | Here we use Equation 2: | ||
<center>< | <center><math>\frac{M_{F}}{M_{P}} = \frac{0.038-0.002}{0.00711-0.002}</math></center> | ||
<center>< | <center><math>\frac{M_{F}}{M_{P}} = 7.045</math></center> | ||
<center>< | <center><math>M_{F} = 7.045(20kg)</math></center> | ||
<center>< | <center><math>M_{F} = 140.9 kg</math></center> | ||
Therefore we would need 140.9 kg of feed uranium at a concentration of 0.711% in order to produce 20 kg of 3.8% enriched uranium. | Therefore we would need 140.9 kg of feed uranium at a concentration of 0.711% in order to produce 20 kg of 3.8% enriched uranium. | ||
| Line 56: | Line 56: | ||
Here we first use Equation 3 for each of the concentration percentages and plug what we get into Equation 4: | Here we first use Equation 3 for each of the concentration percentages and plug what we get into Equation 4: | ||
<center>< | <center><math>V(x_{P}) = (1 - 2(0.038))\ln\left(\frac{1 - 0.038}{0.038}\right)</math></center> | ||
<center>< | <center><math>V(x_{P}) = 2.986</math></center> | ||
Do this for the other two concentrations and find the values equal: V(x<sub>F</sub>)= 4.869, V(x<sub>T</sub>)= 6.188. Then plug these three value functions into Equation 4 to find: | Do this for the other two concentrations and find the values equal: V(x<sub>F</sub>)= 4.869, V(x<sub>T</sub>)= 6.188. Then plug these three value functions into Equation 4 to find: | ||
<center>< | <center><math>SWU = 20kg(2.986) + 120.9kg(6.188) - 140.9kg(4.865)</math></center> | ||
<center>< | <center><math>SWU = 121.807 kg</math></center> | ||
We can then multiply this number by both the kWh and cost per SWU to find our two answers: | We can then multiply this number by both the kWh and cost per SWU to find our two answers: | ||
<center>< | <center><math>(121.807kg)(50kWh/1kg) = 6090.355 kWh</math></center> | ||
And | And | ||
<center>< | <center><math>(121.807kg)(\$130.75/1kg) = \$15926.27</math></center> | ||
Therefore it would take 6090.355 kWh to produce this uranium at a cost of $15926.27. | Therefore it would take 6090.355 kWh to produce this uranium at a cost of $15926.27. | ||
| Line 81: | Line 81: | ||
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
[[Category: Uploaded]] | |||
Revision as of 22:34, 11 May 2018
The Separative Work Unit (SWU) is a unit that defines the effort required in the uranium enrichment process, in which uranium-235 and -238 are separated. The separative work unit is measured in units of kg (kilograms) and can then be manipulated to determine cost per SWU and kWh (kilowatt hour) per SWU (it is not work in the traditional physics sense).
Natural uranium is 0.711% 235U and needs to be enriched to between 3 and 5% for use in a light water reactor (a BWR or a PWR). In order to do this, the uranium undergoes an enrichment process that requires an input of separative work. This is due to the second law of thermodynamics which states that the entropy (disorder) of any isolated system must increase; but because separated isotopes represent more order (lower entropy) than mixed isotopes, effort must be put in to enrich the uranium.[2]
Calculating SWU
The amount of uranium required to be fed into the enrichment plant in order to obtain a desired amount of enriched product depends on the desired enrichment of the product, the original enrichment of the feed and the enrichment of the depleted uranium or the 'tails'. Knowing this, a mass balance equation using these three masses and their respective percent concentrations can be written:[2]
Where xF is the concentration of the feed uranium and MF is the mass of the feed uranium, xP is the concentration of the product uranium and MP is the mass of the product uranium, xT is the concentration of the tails and MT is the mass of the tails.
Applying conservation of mass (MF = MP + MT) and manipulating the equation a little bit we're left with:[2]
The concentration of the feed uranium will almost always be 0.711%, which is the concentration of natural uranium, therefore as long as the desired concentration of product and tails along with desired mass of product are known, mass of feed can be determined.
Separative work can be expressed in terms of a function V(x) known as the value function, given by:[2]
Where x is the enrichment concentration.
The SWU can then be determined using the masses of each uranium concentration found using equation 2 and the value function found for each respective concentration:[2]
For a better understanding of SWU, a online SWU calculator can be used.
Example Calculation
You want to make 20 kg of uranium enriched to 3.8% 235U by weight. Your tailing fraction is 0.2% by weight. You can produce 1 kg SWU for 50 kWhr at a cost of $130.75 per 1 kg SWU.
How much feed uranium do you need?
Here we use Equation 2:
Therefore we would need 140.9 kg of feed uranium at a concentration of 0.711% in order to produce 20 kg of 3.8% enriched uranium.
How much energy does it take to make this uranium and what is the cost?
Here we first use Equation 3 for each of the concentration percentages and plug what we get into Equation 4:
Do this for the other two concentrations and find the values equal: V(xF)= 4.869, V(xT)= 6.188. Then plug these three value functions into Equation 4 to find:
We can then multiply this number by both the kWh and cost per SWU to find our two answers:
And
Therefore it would take 6090.355 kWh to produce this uranium at a cost of $15926.27.
References
- ↑ World Nuclear Association. (June 28, 2016). Uranium Enrichment [Online]. Available: http://www.world-nuclear.org/information-library/nuclear-fuel-cycle/conversion-enrichment-and-fabrication/uranium-enrichment.aspx
- ↑ 2.0 2.1 2.2 2.3 2.4 John R. Lamarsh, Anthony J. Baratta. (June 28, 2016). Introduction to Nuclear Engineering. Third Edition. Upper Saddle River, NJ, U.S.A:Prentice Hall, 2001.