The pH scale: Difference between revisions
J.williams (talk | contribs) No edit summary |
J.williams (talk | contribs) m (1 revision imported) |
(No difference)
| |
Revision as of 21:31, 26 August 2015
The term pH is an abbreviation for power of Hydrogen, which is a measure of the acidity or alkalinity (how base something is) of a chemical solution. Just like hot and cold are variations of temperature, acidic and basic describe variations of chemical composition. Mixing an acid and base will balance out the acidity, just like mixing hot and cold water will balance out the temperature; a substance that is neither an acid nor a base is called "neutral".[2]
Acids and bases are very important in the world, and can be found almost everywhere. One main example in the context of energy is acid rain, which is formed when water interacts with various pollutants in the atmosphere. Acid rain is a concern of pollution and has many adverse effects on ecosystems and the environment. Read more about acid rain here.
The pH scale
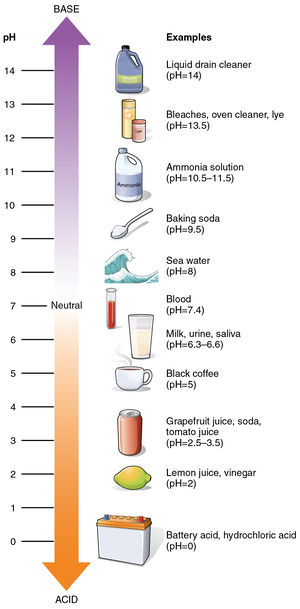
The pH scale measures the concentration of hydrogen ions (H+) within a substance; the larger the H+ concentration (number of molecules in a given volume), the lower the pH. Conversely, the larger the concentration of hydroxide ions (OH-), the higher the pH.[3] The pH scale ranges from 0 to 14 (extreme values are -1 and 15, but these are rarely encountered), with acids being defined as having a pH less than 7, and bases being defined as having a pH greater than 7. This makes 7 neutral, which is the pH of pure water. The reason pure water is neutral is because the concentration of H+ and OH- is exactly the same, therefore cancelling out.
The scale (Figure 1) is defined on a base 10 logarithmic scale, meaning that any 1 digit difference is 10 times more acidic or basic. For example, a pH of 1.0 is 10 times more acidic than a pH of 2.0, and it is 100 times more acidic than a pH of 3.0!
To explore pH further please see the UC Davis chem wiki. Also, the PhET simulation below was graciously provided by the University of Colorado. This simulation can help explore how pH changes as liquids mix.
References
- ↑ Wikimedia Commons [Online], Available: https://upload.wikimedia.org/wikipedia/commons/2/23/216_pH_Scale-01.jpg
- ↑ US EPA. (July 8, 2015). What is pH? [Online], Available: http://www.epa.gov/acidrain/measure/ph.html
- ↑ Engineering Toolbox. (July 8, 2015). pH Definition [Online], Available: http://www.engineeringtoolbox.com/ph-d_483.html

