Supercapacitor
Supercapacitors are a new type of capacitor, also known as ultra-capacitors. The characteristics of supercapacitors give them a higher capacitance than conventional capacitors. Supercapacitors have a higher power density, but they suffer from low energy densities compared to batteries.[1] However, recent advances in carbon-based materials, namely graphene, increase the energy density to nearly the level of batteries.[2]
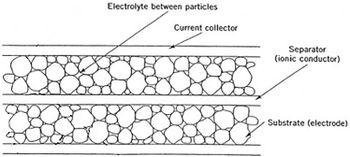
A supercapacitor is a modified capacitor. One modification is the electrode is coated or made of a porous material.[4][5] Being porous increases the surface area without changing the size of the capacitor, allowing it to hold more charge.
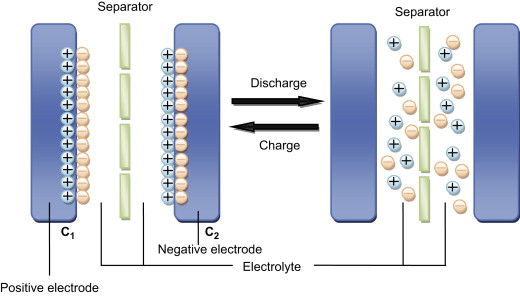
Supercapacitors make use of an electrolyte, a substance made of positively and negatively charged particles. When the electrodes are charged, the electrolyte polarizes according to the charge of the electrodes. This separation of charge creates two oppositely charged layers that act like new charged plates. These four "plates" act like two conventional capacitors attached in series. These capacitors have plate separation distances that are a few nanometers thick.[3]
The combination of the increased surface area and the small plate separation distance increases the capacitance and the energy stored in the supercapacitor.
The cross section to the right shows a separator between the electrodes. The separator stops the electrodes from touching and short ciruiting.
Materials
The popular material to use for supercapacitors electrodes is carbon. It can be in the form of graphite, carbon nanofibres, activated carbon, and recently, graphene. These materials are electrically conductive and have or can be engineered to have porous surfaces.[7], which allow them to store more electricity.
Supercapacitors can use a variety of electrolyte solutions. These include acidic, alkaline, and organic electrolytes. Alkaline and acid electrolytes generally achieve higher specific capacitance,[8] where as organic electrolytes are capable of sustaining a higher operating voltage.[9]
Applications
Electric double-layer capacitors (EDLCs) are a type of supercapacitors which is the type that we focus on here.
Currently, EDLCs cannot store much energy, so they have been limited to small energy burst usages. An example is electric vehicle acceleration. Batteries do not do well with fast changes in power output. So, the capacitor is used to provide the necessary power needed for acceleration.
An EDLC does not need to be used with a battery. In Hong Kong, the city is testing buses that run only on supercapacitors. Supercapacitors are considered perfect for buses because buses travel small distances and stop frequently.[10][11] As the bus slows down, regenerative braking can be used to charge a series of supercapacitors. This stores enough energy for the bus to travel to the next stop. The capacitors will discharge as the bus travels, and then they will recharges when as the bus slows down at the next stop.
References
- ↑ M. Jayalakshmi and K. Balasubramanian, "Simple to supercapacitors-an overview," Int. J. Electrochem. Sci., vol. 3, no. 11, pp. 1196-1217, Nov. 2008.
- ↑ M.D. Stoller et al., "Graphene-based Ultracapacitors," Nano Lett., vol. 8, no. 10, pp. 3498-3502, Oct. 2008.
- ↑ 3.0 3.1 A. Burke, “Ultracapacitors: why, how, and where is the technology,” J. Power Sources, vol. 91, no. 1, pp. 37-50, Nov. 2000.
- ↑ L. Oakes et al., “Surface engineered porous silicon for stable, high performance electrochemical supercapacitors,” Sci. Rep., vol. 3., pp. 1-7, Oct. 2013.
- ↑ M. F. El-Kady et al., “Laser scribing of high-performance and flexible graphene-based electrochemical capacitors,” Science, vol. 335, no. 6074, pp. 1326-1330, Mar. 2012.
- ↑ X. Li and B. Wei, "Supercapacitors based on nanostructured carbon," Nano Energy, vol. 2, no. 2, pp. 159-173, Mar. 2013.
- ↑ P. Simon and Y. Gogotsi, “Materials for electrochemical capacitors,” Nature Mater., vol. 7, no. 11, pp. 845-854, Nov. 2008.
- ↑ A. G. Pandolfo and A. F. Hollenkamp, "Carbon properties and their role in supercapacitors," J. Power Sources, vol. 157, no. 1, pp. 11-27, Jun. 2006.
- ↑ P. Simon and Y. Gogotsi, "Materials for electrochemical capacitors," Nat. Mater., vol. 7, no. 11, pp. 845-854, Nov. 2008.
- ↑ Maxwell Technologies. (2013). Maxwell Ultracapacitor Transportation Solutions [Online]. Available: http://www.maxwell.com/products/ultracapacitors/industries/transportation
- ↑ T. Hamilton. (2009, October 19). Next Stop: Ultracapacitor Buses [Online]. Available: http://www.technologyreview.com/news/415773/next-stop-ultracapacitor-buses/

