Valence and core electrons
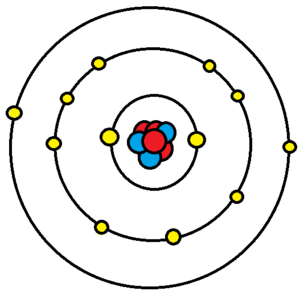
Electrons exist in orbitals around a nucleus. These orbitals and the energy needed to remove each of these electrons from the atom are set by quantum mechanics. Each of these orbitals serves to create a shell of electrons in the atom. Valence electrons are the electrons orbiting the nucleus in the outermost atomic shell of an atom. Electrons that are closer to the nucleus are in filled orbitals and are called core electrons. Valence electrons are the farthest from the positive charge (the protons) and thus tend to be easier to remove than core electrons; this means that it takes them less energy to move far away from the atom. This difference comes from the electric force being an inverse square law. In addition, core electrons in the inner shells have lower energy levels than the valence electrons occupying the outer shell. This means that electrons in the inner shells can absorb bits of energy and move (jump) to the valence electron shell.
Valence electrons and chemical reactions
In chemical reactions, the electrons can even break free from the valence shell. This can be to create an ionic bond or to become an ion. When an electron leaves a neutral atom, it loses a negative charge and turns into a positively charged ion. For example, sodium (Na) has one electron in it's outer shell. Thus it wants to lose a single electron and become an Na+ ion. An atom can also gain an electron (usually to fill it's valence shell) and turn into a negatively charged ion. This can be seen with Chlorine, which in it's neutral state is missing one electron in it's valence electron shell. Thus, it wants to pick up an electron and become a Cl- ion.[2]
Noble gases are elements that have a full valence shell, meaning that the outer shell is completely filled with electrons. Noble gases neither want to gain or lose an electron, which means they tend to be chemically inert (unreactive). In general, atoms want to have full valence electron shells. This is why atoms, and also chemical compounds, lose or gain electrons to become ions, and also why they form ionic and covalent bonds.
Valence electrons and chemical bonding
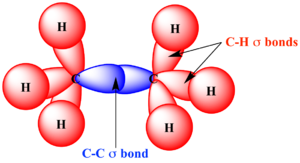
In both ionic and covalent bonding, it is the valence electrons that participate these chemical bonds.[2] In single covalent bonds, typically both atoms in the bond contribute one valence electron each in order to form a shared electron pair. An example is seen in figure 2 where a strong sigma bond (σ bond) is formed. Valence electrons are also used to form double and triple bonds, which have valence electrons configured in pi bonds (π bonds).[4]
In ionic bonding, valence electrons are completely transferred between atoms. It is a type of chemical bond that generates two oppositely charged ions, one anion and one cation. Ionic bonding is observed because metals have few electrons in their valence orbitals and nonmetals almost have 8 electrons in their valence shells. Metals lose their valence electrons and become more stable by satisfying the octet rule. Similarly, nonmetals will readily accept electrons to achieve a noble gas configuration.[4]
For more information about valence electrons, core electrons and how they related to chemical reactions please see UC Davis's chem wiki.
For Further Reading
- Atom
- Electron
- Ion
- Nucleus
- Or explore a random page
References
- ↑ This image was created by part of the Energy Education team.
- ↑ 2.0 2.1 "Valence electrons and open valences." Chemistry LibreTexts, 2019. [Online]. Available: https://chem.libretexts.org/@go/page/16945. [Accessed: May 15, 2021]
- ↑ "Illustrated Glossary of Organic Chemistry." UCLA College. Online. Available: http://www.chem.ucla.edu/~harding/IGOC/S/sigma_bond.html.
- ↑ 4.0 4.1 "Ionic and Covalent Bonds," Chemistry LibreTexts, 2020. Online. Available: https://chem.libretexts.org/@go/page/839.