Base (chemistry): Difference between revisions
J.williams (talk | contribs) m (1 revision imported) |
No edit summary |
||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2018-07-20]] | ||
[[File:bleachs.JPG|160px|thumb|Figure 1. Bleach has a pH of around 12-13.]] | [[File:bleachs.JPG|160px|thumb|Figure 1. Bleach has a pH of around 12-13.]] | ||
<onlyinclude>A '''base''' or '''alkaline''' is | <onlyinclude>A '''base''' or '''alkaline''' is any substance that will react with water to produce OH<sup>-</sup><ref name=libre>Chemistry LibreTexts. (July 11 2018). ''Overview of Acids and Bases'' [online], Available:https://chem.libretexts.org/Textbook_Maps/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Acids_and_Bases/Acid/Overview_of_Acids_and_Bases </ref> Generally, solutions of bases in water will have a [[pH]] greater than 7. | ||
In water, there is always some H<sup>+</sup> and OH<sup>-</sup> [[ion]]s in solution due to the self-ionization of water. In basic solutions, there will be more OH<sup>-</sup> than H<sup>+</sup>. More concentrated bases will have more OH<sup>-</sup> in solution. Following the pH scale, a more basic solution - one with more OH<sup>-</sup> - will have a <em>higher</em> pH value. </onlyinclude>[[Acid]]s can react with bases in a [[neutralization]] reaction, where the H<sup>+</sup> from the acid reacts with the OH<sup>-</sup> of the base to produce a solution with a lower OH<sup>-</sup> concentration - and a lower pH - than the original base solution. | |||
Most common inorganic bases are hydroxides (containing OH<sup>-</sup> in their composition), such as sodium hydroxide, NaOH, or calcium hydroxide, Ca(OH)<sub>2</sub>. However some [[organic molecule|organic]] bases exist, such as ammonia-based molecules like amines, that do not contain OH<sup>-</sup> directly: any OH<sup>-</sup> in a solution of these molecules is formed from a reaction with water. <ref name=libre3>Chemistry LibreTexts. (July 11, 2018). ''Organic Acids and Organic Bases'' [online], Available: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Map%3A_Organic_Chemistry_(McMurry)/chapter_02%3A_Polar_Covalent_Bonds%3B_Acids_and_Bases/2.10_Organic_Acids_and_Organic_Bases#Ammonia_as_a_weak_base </ref> | |||
===Uses=== | ===Uses=== | ||
The ability of bases to [[neutralization|neutralize]] acids is very useful, as many reactions and industrial processes have acidic waste products. Some examples of using bases to neutralizing unwanted acids: | |||
*[[Acid rain]] can be neutralized by basic minerals, such as limestone. (Limestone is mainly calcium carbonate.<ref name=geo>Geology.com. (July 11, 2018). ''Limestone'' [online], Available: https://geology.com/rocks/limestone.shtml</ref>) | |||
*[[Scrubber]]s used in [[power plant]]s use bases (e.g. calcium hydroxide) in order to reduce the release of [[sulfur oxides]] (a type of acidic [[pollutant]]) produced by the plant. | |||
*Antacids contain bases (most often calcium carbonate) that react with stomach acid in order to treat heartburn and indigestion. <ref name=libre2>Chemistry Libretexts. (July 11, 2018). ''Heartburn'' [online], Available: https://chem.libretexts.org/Textbook_Maps/General_Chemistry/Map%3A_A_Molecular_Approach_(Tro)/16%3A_Acids_and_Bases/16.01%3A_Heartburn</ref> | |||
Outside of neutralization, bases are also used in chemical reactions: | |||
* | |||
*Sodium | *Sodium hydroxide and potassium hydroxide are used to make soaps. <ref name=soap>Ogden Publications Inc. (July 11, 2018). ''Mother Earth Living: Honey and Beeswax Soap Recipe'' [online], Available: https://www.motherearthliving.com/health-and-wellness/beauty-recipes/honey-and-beeswax-soap-recipe-ze0z1506zdeb</ref> | ||
*Sodium | *Sodium hypochlorite (NaOCl) is the main ingredient in laundry bleach. <ref name=bleach>Chlorox. (July 11, 2018). ''What is bleach?'' [online], Available: https://www.clorox.com/how-to/laundry-basics/bleach-101/bleach/ </ref> | ||
*Sodium bicarbonate (baking soda) is used to generate gases for leavening bread and baked goods, and in some fire extinguishers. <ref name=fire>Piper Fire Protection Inc. (July 11, 2018). ''Types of Fire Extinguishers and What They Do''. [online]. Available: http://www.piperfire.com/types-of-fire-extinguishers-and-what-they-do/</ref> | |||
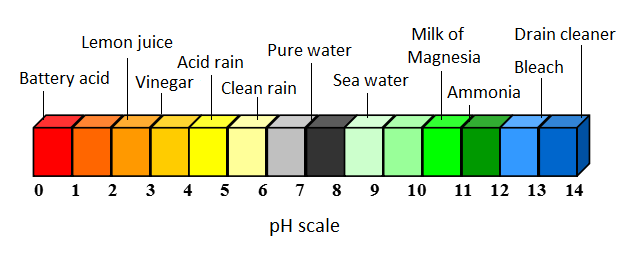
[[File:pHacid.png|800px|thumb|center|Figure 2. Various acids and bases on the pH scale.<ref>Adapted from Energy: Its use and the Environment -- R. A. Hinrichs and M. Kleinbach, "Acid Rain," in ''Energy: Its Use and the Environment'', 5th ed. Toronto, Ont. Canada: Brooks/Cole, 2006, ch.8, sec.C, pp.252-256</ref>]] | [[File:pHacid.png|800px|thumb|center|Figure 2. Various acids and bases on the pH scale.<ref>Adapted from Energy: Its use and the Environment -- R. A. Hinrichs and M. Kleinbach, "Acid Rain," in ''Energy: Its Use and the Environment'', 5th ed. Toronto, Ont. Canada: Brooks/Cole, 2006, ch.8, sec.C, pp.252-256</ref>]] | ||
To learn more about acids and bases please see [http:// | To learn more about acids and bases please see [https://chem.libretexts.org/Textbook_Maps/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Acids_and_Bases/Acid/Overview_of_Acids_and_Bases the Chemistry LibreText]. | ||
==Phet Simulation== | |||
The [http://phet.colorado.edu/ University of Colorado] has graciously allowed us to use the following Phet simulation. Explore this simulation to see how the pH of acids and bases work. | |||
<html> | |||
<iframe src="https://phet.colorado.edu/sims/html/acid-base-solutions/latest/acid-base-solutions_en.html" width="800" height="600"></iframe> | |||
</html> | |||
==For Further Reading== | |||
*[[Acid]] | |||
*[[The pH scale]] | |||
*[[Chemical]] | |||
*[[Water]] | |||
*[[Acid rain]] | |||
*[[Water cycle]] | |||
*Or explore a [[Special:Random|random page]] | |||
To learn more about acids and bases please see [https://chem.libretexts.org/Textbook_Maps/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Acids_and_Bases/Acid/Overview_of_Acids_and_Bases the Chemistry LibreText]. | |||
==References== | ==References== | ||
{{ | {{reflist}} | ||
Revision as of 02:04, 13 July 2018
A base or alkaline is any substance that will react with water to produce OH-[1] Generally, solutions of bases in water will have a pH greater than 7.
In water, there is always some H+ and OH- ions in solution due to the self-ionization of water. In basic solutions, there will be more OH- than H+. More concentrated bases will have more OH- in solution. Following the pH scale, a more basic solution - one with more OH- - will have a higher pH value. Acids can react with bases in a neutralization reaction, where the H+ from the acid reacts with the OH- of the base to produce a solution with a lower OH- concentration - and a lower pH - than the original base solution.
Most common inorganic bases are hydroxides (containing OH- in their composition), such as sodium hydroxide, NaOH, or calcium hydroxide, Ca(OH)2. However some organic bases exist, such as ammonia-based molecules like amines, that do not contain OH- directly: any OH- in a solution of these molecules is formed from a reaction with water. [2]
Uses
The ability of bases to neutralize acids is very useful, as many reactions and industrial processes have acidic waste products. Some examples of using bases to neutralizing unwanted acids:
- Acid rain can be neutralized by basic minerals, such as limestone. (Limestone is mainly calcium carbonate.[3])
- Scrubbers used in power plants use bases (e.g. calcium hydroxide) in order to reduce the release of sulfur oxides (a type of acidic pollutant) produced by the plant.
- Antacids contain bases (most often calcium carbonate) that react with stomach acid in order to treat heartburn and indigestion. [4]
Outside of neutralization, bases are also used in chemical reactions:
- Sodium hydroxide and potassium hydroxide are used to make soaps. [5]
- Sodium hypochlorite (NaOCl) is the main ingredient in laundry bleach. [6]
- Sodium bicarbonate (baking soda) is used to generate gases for leavening bread and baked goods, and in some fire extinguishers. [7]
To learn more about acids and bases please see the Chemistry LibreText.
Phet Simulation
The University of Colorado has graciously allowed us to use the following Phet simulation. Explore this simulation to see how the pH of acids and bases work.
For Further Reading
- Acid
- The pH scale
- Chemical
- Water
- Acid rain
- Water cycle
- Or explore a random page
To learn more about acids and bases please see the Chemistry LibreText.
References
- ↑ Chemistry LibreTexts. (July 11 2018). Overview of Acids and Bases [online], Available:https://chem.libretexts.org/Textbook_Maps/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Acids_and_Bases/Acid/Overview_of_Acids_and_Bases
- ↑ Chemistry LibreTexts. (July 11, 2018). Organic Acids and Organic Bases [online], Available: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Map%3A_Organic_Chemistry_(McMurry)/chapter_02%3A_Polar_Covalent_Bonds%3B_Acids_and_Bases/2.10_Organic_Acids_and_Organic_Bases#Ammonia_as_a_weak_base
- ↑ Geology.com. (July 11, 2018). Limestone [online], Available: https://geology.com/rocks/limestone.shtml
- ↑ Chemistry Libretexts. (July 11, 2018). Heartburn [online], Available: https://chem.libretexts.org/Textbook_Maps/General_Chemistry/Map%3A_A_Molecular_Approach_(Tro)/16%3A_Acids_and_Bases/16.01%3A_Heartburn
- ↑ Ogden Publications Inc. (July 11, 2018). Mother Earth Living: Honey and Beeswax Soap Recipe [online], Available: https://www.motherearthliving.com/health-and-wellness/beauty-recipes/honey-and-beeswax-soap-recipe-ze0z1506zdeb
- ↑ Chlorox. (July 11, 2018). What is bleach? [online], Available: https://www.clorox.com/how-to/laundry-basics/bleach-101/bleach/
- ↑ Piper Fire Protection Inc. (July 11, 2018). Types of Fire Extinguishers and What They Do. [online]. Available: http://www.piperfire.com/types-of-fire-extinguishers-and-what-they-do/
- ↑ Adapted from Energy: Its use and the Environment -- R. A. Hinrichs and M. Kleinbach, "Acid Rain," in Energy: Its Use and the Environment, 5th ed. Toronto, Ont. Canada: Brooks/Cole, 2006, ch.8, sec.C, pp.252-256


