Acid: Difference between revisions
J.williams (talk | contribs) m (1 revision imported) |
m (1 revision imported) |
||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2018-07-20]] | ||
[[Category:Translated to French]] | |||
[[fr:Acide]] | |||
[[File:Lemon.jpg|250px|thumb|Figure 1. Lemons are acidic, with a pH of around 2.<ref>Wikimedia Commons [Online], Available: https://commons.wikimedia.org/wiki/File:Lemon.jpg</ref>]] | [[File:Lemon.jpg|250px|thumb|Figure 1. Lemons are acidic, with a pH of around 2.<ref>Wikimedia Commons [Online], Available: https://commons.wikimedia.org/wiki/File:Lemon.jpg</ref>]] | ||
<onlyinclude>An '''acid''' is any substance that will react with water to produce H<sup>+</sup> <ref name=libre>Chemistry LibreTexts. (July 11 2018). ''Overview of Acids and Bases'' [online], Available: https://chem.libretexts.org/Textbook_Maps/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Acids_and_Bases/Acid/Overview_of_Acids_and_Bases </ref> (H<sup>+</sup> reacts in water to produce H<sub>3</sub>O<sup>+</sup> - both can be treated as equivalent.) Generally, solutions of acid in water will have a [[pH]] less than 7.</onlyinclude> | |||
< | In water, there are always some H<sup>+</sup> and OH<sup>-</sup> [[ion]]s in solution due to the self-ionization of water, but in acidic solutions, there will be more H<sup>+</sup> than OH<sup>-</sup>. More concentrated acids will have more H<sup>+</sup> in solution. Following the pH scale, a more acidic solution - one with more H<sup>+</sup> - will have a <em>lower</em> pH value. | ||
Acids can react with [[base]]s in a [[neutralization]] reaction, where the H<sup>+</sup> from the acid reacts with the OH<sup>-</sup> of the base to produce a solution with a lower H<sup>+</sup> concentration - and a higher pH. | |||
[[Acid rain]] is an environmental problem caused by [[pollution]] | [[Acid rain]] is an environmental problem, caused by acidic substances dissolving in rainwater. Often, these acidic substances are from [[pollution]] in the atmosphere. Acid rain, acid snow, and acid fog (all collectively referred to as 'acid rain') can all form from the interaction of water vapour, rain, or fog with acidic [[pollutant]]s. Acid rain can be quite damaging due to its adicity - clean rain has a typical pH of around 5.6, while acid rain has a typical pH of about 4.3: in other words, acid rain is usually about 10 times more acidic than clean rain. <ref name=EPA>United States Environmental Protection Agency. (July 11 2018). ''What is Acid Rain'' [online], Available: https://www.epa.gov/acidrain/what-acid-rain </ref> Visit the [[acid rain|acid rain page]] for more information. | ||
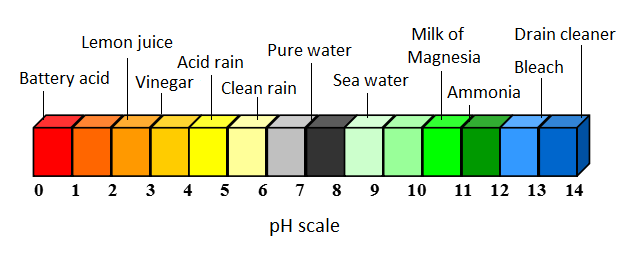
[[File:pHacid.png|800px|thumb|center|Figure 2. Various acids and bases on the pH scale.<ref>Adapted from Energy: Its use and the Environment -- R. A. Hinrichs and M. Kleinbach, "Acid Rain," in ''Energy: Its Use and the Environment'', 5th ed. Toronto, Ont. Canada: Brooks/Cole, 2006, ch.8, sec.C, pp.252-256</ref>]] | [[File:pHacid.png|800px|thumb|center|Figure 2. Various acids and bases on the pH scale.<ref>Adapted from Energy: Its use and the Environment -- R. A. Hinrichs and M. Kleinbach, "Acid Rain," in ''Energy: Its Use and the Environment'', 5th ed. Toronto, Ont. Canada: Brooks/Cole, 2006, ch.8, sec.C, pp.252-256</ref>]] | ||
| Line 12: | Line 15: | ||
==Uses== | ==Uses== | ||
Acids | Acids occur naturally (for example in fruits and vegetables) and man-made acids are widely used in industry, for cleaning, and as food additives. For example, they can be used to remove [[oxidize|rust]] from [[metal]]s, as an [[electrolyte]] in a wet-cell [[battery]] (such as a car battery), in the chemical industry as an important component of production, and as additives to food and drink to alter taste and act as a preservative (e.g. in soda). | ||
Acids are also important in the human body. | Acids are also important in the human body. Stomach acid breaks down food in one of the first stages of digestion, and [[organic molecule|organic]] acids are used for [[protein]] synthesis, tissue repair, and pH balancing in tissues. Ascorbic acid (Vitamin C) is essential for the human body, as well as many other acids. The "A" in DNA and RNA stands for acid, and these are crucial [[molecule]]s for all life. | ||
==Phet Simulation== | |||
The [http://phet.colorado.edu/ University of Colorado] has graciously allowed us to use the following Phet simulation. Explore this simulation to see how the pH of acids and bases work. | |||
To learn more about acids and bases please see [ | <html> | ||
<iframe src="https://phet.colorado.edu/sims/html/acid-base-solutions/latest/acid-base-solutions_en.html" width="800" height="600"></iframe> | |||
</html> | |||
==For Further Reading== | |||
*[[Base]] | |||
*[[The pH scale]] | |||
*[[Chemical]] | |||
*[[Water]] | |||
*[[Acid rain]] | |||
*[[Water cycle]] | |||
*Or explore a [[Special:Random|random page]] | |||
To learn more about acids and bases please see [https://chem.libretexts.org/Textbook_Maps/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Acids_and_Bases/Acid/Overview_of_Acids_and_Bases the Chemistry LibreText]. | |||
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
Latest revision as of 00:03, 27 September 2021

An acid is any substance that will react with water to produce H+ [2] (H+ reacts in water to produce H3O+ - both can be treated as equivalent.) Generally, solutions of acid in water will have a pH less than 7.
In water, there are always some H+ and OH- ions in solution due to the self-ionization of water, but in acidic solutions, there will be more H+ than OH-. More concentrated acids will have more H+ in solution. Following the pH scale, a more acidic solution - one with more H+ - will have a lower pH value.
Acids can react with bases in a neutralization reaction, where the H+ from the acid reacts with the OH- of the base to produce a solution with a lower H+ concentration - and a higher pH.
Acid rain is an environmental problem, caused by acidic substances dissolving in rainwater. Often, these acidic substances are from pollution in the atmosphere. Acid rain, acid snow, and acid fog (all collectively referred to as 'acid rain') can all form from the interaction of water vapour, rain, or fog with acidic pollutants. Acid rain can be quite damaging due to its adicity - clean rain has a typical pH of around 5.6, while acid rain has a typical pH of about 4.3: in other words, acid rain is usually about 10 times more acidic than clean rain. [3] Visit the acid rain page for more information.
Uses
Acids occur naturally (for example in fruits and vegetables) and man-made acids are widely used in industry, for cleaning, and as food additives. For example, they can be used to remove rust from metals, as an electrolyte in a wet-cell battery (such as a car battery), in the chemical industry as an important component of production, and as additives to food and drink to alter taste and act as a preservative (e.g. in soda).
Acids are also important in the human body. Stomach acid breaks down food in one of the first stages of digestion, and organic acids are used for protein synthesis, tissue repair, and pH balancing in tissues. Ascorbic acid (Vitamin C) is essential for the human body, as well as many other acids. The "A" in DNA and RNA stands for acid, and these are crucial molecules for all life.
Phet Simulation
The University of Colorado has graciously allowed us to use the following Phet simulation. Explore this simulation to see how the pH of acids and bases work.
For Further Reading
- Base
- The pH scale
- Chemical
- Water
- Acid rain
- Water cycle
- Or explore a random page
To learn more about acids and bases please see the Chemistry LibreText.
References
- ↑ Wikimedia Commons [Online], Available: https://commons.wikimedia.org/wiki/File:Lemon.jpg
- ↑ Chemistry LibreTexts. (July 11 2018). Overview of Acids and Bases [online], Available: https://chem.libretexts.org/Textbook_Maps/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Acids_and_Bases/Acid/Overview_of_Acids_and_Bases
- ↑ United States Environmental Protection Agency. (July 11 2018). What is Acid Rain [online], Available: https://www.epa.gov/acidrain/what-acid-rain
- ↑ Adapted from Energy: Its use and the Environment -- R. A. Hinrichs and M. Kleinbach, "Acid Rain," in Energy: Its Use and the Environment, 5th ed. Toronto, Ont. Canada: Brooks/Cole, 2006, ch.8, sec.C, pp.252-256

