Chemical isomer: Difference between revisions
J.williams (talk | contribs) No edit summary |
m (1 revision imported) |
||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2020-01-31]] | ||
<onlyinclude>'''Chemical isomers''' are [[molecule]]s of the same [[chemical]] composition, but different molecular structure. In other words, two isomers will have the same number and type of [[atom]]s, but a different arrangement in space.</onlyinclude> Although these molecules are of the same composition, this different arrangement can result in a difference of physical and chemical properties (such as [[boiling point]], [[melting point]], etc).<ref>Compound Interest, ''A Bried Guide to Types of Isomerism in Organic Chemisty'' [Online], Available: http://www.compoundchem.com/2014/05/22/typesofisomerism/</ref> | <onlyinclude>'''Chemical isomers''' are [[molecule]]s of the same [[chemical]] composition, but different molecular structure. In other words, two isomers will have the same number and type of [[atom]]s, but a different arrangement in space.</onlyinclude> Although these molecules are of the same composition, this different arrangement can result in a difference of physical and chemical properties (such as [[boiling point]], [[melting point]], etc).<ref>Compound Interest, ''A Bried Guide to Types of Isomerism in Organic Chemisty'' [Online], Available: http://www.compoundchem.com/2014/05/22/typesofisomerism/</ref> | ||
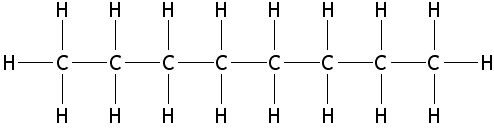
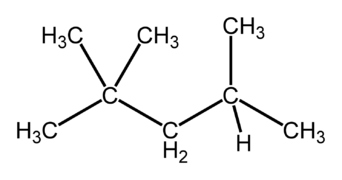
Isomers are very important for [[fuel]] choices when providing [[energy]] through [[combustion]]. Isomers of compounds burn differently, such as [[octane]], which has 18 isomers.<ref name=e>eHow, ''Importance of Isomers in Fuels'' [Online], Available: http://www.ehow.com/about_5587676_importance-isomers-fuels.html</ref> For most applications the goal when burning a fuel is to have a stable burn, and certain isomers accomplish this better. For octane, the more linear isomers as visible in Figure 1 burn much quicker than isomers that are "branched" like in Figure 2. The branched structures allow the fuel to burn slowly and evenly, therefore they are of more desire.<ref name=e/> [[Octane rating]], which is the number visible on gas pumps, is a measure of how much iso-octane (Figure 2) is in a fuel mixture. The more iso-octane present in the mixture, the better the burn. | Isomers are very important for [[fuel]] choices when providing [[energy]] through [[combustion]]. Isomers of compounds burn differently, such as [[octane]], which has 18 isomers.<ref name=e>eHow, ''Importance of Isomers in Fuels'' [Online], Available: http://www.ehow.com/about_5587676_importance-isomers-fuels.html</ref> For most applications the goal when burning a fuel is to have a stable burn, and certain isomers accomplish this better. For octane, the more linear isomers as visible in Figure 1 burn much quicker than isomers that are "branched" like in Figure 2. The branched structures allow the fuel to burn slowly and evenly, therefore they are of more desire.<ref name=e/> [[Octane rating]], which is the number visible on gas pumps, is a measure of how much iso-octane (Figure 2) is in a fuel mixture. The more iso-octane present in the mixture, the better the burn. | ||
<gallery mode=packed> | <gallery mode=packed> | ||
| Line 9: | Line 8: | ||
File:Isooctane.png|Figure 2. iso-octane, a more branched isomer of octane, used in most fuels and gives the octane rating for a fuel.<ref>Wikimedia Commons [Online], Available: http://upload.wikimedia.org/wikipedia/commons/7/79/Isooctane.png</ref> | File:Isooctane.png|Figure 2. iso-octane, a more branched isomer of octane, used in most fuels and gives the octane rating for a fuel.<ref>Wikimedia Commons [Online], Available: http://upload.wikimedia.org/wikipedia/commons/7/79/Isooctane.png</ref> | ||
</gallery> | </gallery> | ||
Visit the [http://chemwiki.ucdavis.edu/Inorganic_Chemistry/Coordination_Chemistry/Isomers UC Davis Chemwiki] for a more in depth look at chemical isomers. | |||
==For Further Reading== | |||
*[[Hydrocarbon combustion]] | |||
*[[Octane]] | |||
*[[Octane rating]] | |||
*[[Chemical]] | |||
*Or explore a [[Special:Random|random page]] | |||
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
[[Category:Uploaded]] | [[Category:Uploaded]] | ||
Latest revision as of 05:10, 31 January 2020
Chemical isomers are molecules of the same chemical composition, but different molecular structure. In other words, two isomers will have the same number and type of atoms, but a different arrangement in space. Although these molecules are of the same composition, this different arrangement can result in a difference of physical and chemical properties (such as boiling point, melting point, etc).[1]
Isomers are very important for fuel choices when providing energy through combustion. Isomers of compounds burn differently, such as octane, which has 18 isomers.[2] For most applications the goal when burning a fuel is to have a stable burn, and certain isomers accomplish this better. For octane, the more linear isomers as visible in Figure 1 burn much quicker than isomers that are "branched" like in Figure 2. The branched structures allow the fuel to burn slowly and evenly, therefore they are of more desire.[2] Octane rating, which is the number visible on gas pumps, is a measure of how much iso-octane (Figure 2) is in a fuel mixture. The more iso-octane present in the mixture, the better the burn.
Figure 1. n-Octane, a more linear isomer of octane compared to its other forms.[3]
Figure 2. iso-octane, a more branched isomer of octane, used in most fuels and gives the octane rating for a fuel.[4]
Visit the UC Davis Chemwiki for a more in depth look at chemical isomers.
For Further Reading
- Hydrocarbon combustion
- Octane
- Octane rating
- Chemical
- Or explore a random page
References
- ↑ Compound Interest, A Bried Guide to Types of Isomerism in Organic Chemisty [Online], Available: http://www.compoundchem.com/2014/05/22/typesofisomerism/
- ↑ 2.0 2.1 eHow, Importance of Isomers in Fuels [Online], Available: http://www.ehow.com/about_5587676_importance-isomers-fuels.html
- ↑ Wikimedia Commons [Online], Available: http://commons.wikimedia.org/wiki/File:Octane-in-full.png
- ↑ Wikimedia Commons [Online], Available: http://upload.wikimedia.org/wikipedia/commons/7/79/Isooctane.png