Hydrogen sulfide: Difference between revisions
J.williams (talk | contribs) m (1 revision imported) |
2dev>Jmdonev No edit summary |
||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2018-12-10]] | ||
[[Category: Rudi grade Ashley edit]] | |||
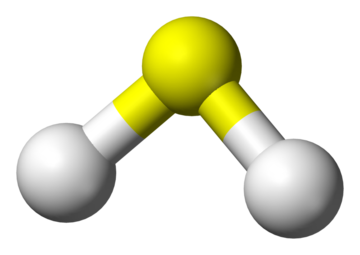
[[File:1024px-Hydrogen-sulfide-3D-balls.png|360px|thumb|right|Figure 1. A ball and stick model of hydrogen sulfide. The white balls are [[hydrogen]] and the yellow ball is [[sulfur]].<ref>Wikimedia Commons. (September 8, 2015). ''Hydrogen Sulfide 3D Balls'' [Online]. Available: https://upload.wikimedia.org/wikipedia/commons/thumb/3/34/Hydrogen-sulfide-3D-balls.png/1024px-Hydrogen-sulfide-3D-balls.png</ref>]] | [[File:1024px-Hydrogen-sulfide-3D-balls.png|360px|thumb|right|Figure 1. A ball and stick model of hydrogen sulfide. The white balls are [[hydrogen]] and the yellow ball is [[sulfur]].<ref>Wikimedia Commons. (September 8, 2015). ''Hydrogen Sulfide 3D Balls'' [Online]. Available: https://upload.wikimedia.org/wikipedia/commons/thumb/3/34/Hydrogen-sulfide-3D-balls.png/1024px-Hydrogen-sulfide-3D-balls.png</ref>]] | ||
<onlyinclude>'''Hydrogen sulfide''' is a [[chemical]] with the formula H<sub>2</sub>S. Hydrogen sulfide is a dangerous, colourless [[gas]] that occurs naturally in many [[petroleum]] products | <onlyinclude>'''Hydrogen sulfide''' is a [[chemical]] with the formula H<sub>2</sub>S. Hydrogen sulfide is a dangerous, colourless [[gas]] that occurs naturally in many [[petroleum]] products. Specifically, it often makes up significant amounts of raw [[natural gas]]. It has a distinct "rotten egg" smell at low concentration levels.</onlyinclude><ref name=pdf>OSHA Fact Sheet. (September 8, 2015). ''Hydrogen Sulfide'' [Online]. Available: https://www.osha.gov/OshDoc/data_Hurricane_Facts/hydrogen_sulfide_fact.pdf</ref> Prolonged exposure will result in olfactory fatigue, which is the loss of the ability to smell it. At high concentrations of hydrogen sulfide, the ability to smell it is lost instantly.<ref name=pdf1>OSHA QuickCard. (Oct 7, 2018). ''Hydrogen Sulfide'' [Online]. Available: https://www.osha.gov/Publications/hydrogen_sulfide.html</ref> In addition to being harmful to human health as an asphyxiant, hydrogen sulfide is both [[flammable]] and explosive. | ||
Hydrogen sulfide can | Hydrogen sulfide exists in natural deposits or can be produced by human activities. For example, hydrogen sulfide occurs naturally in [[crude oil]], natural gas, and in hot springs. Bacterial breakdown of human and animal waste also produced hydrogen sulfide. Humans release hydrogen sulfide through petroleum drilling and refining, wastewater treatment, and paper mills.<ref name=pdf/> | ||
Some additional properties of hydrogen sulfide are outlined in the table below. | Some additional properties of hydrogen sulfide are outlined in the table below. | ||
| Line 19: | Line 20: | ||
|} | |} | ||
The hydrogen sulfide content of natural gas is one of the main safety concerns associated with the use of | The hydrogen sulfide content of natural gas is one of the main safety concerns associated with the use of natural gas. Natural gas with especially high levels of hydrogen sulfide is referred to as '''[[sour gas]]'''. Some of this [[sulfur]] can be removed through a "sweetening" process, called the "amine process" or more commonly the "Girdler process". It is used in most gas sweetening operations and is done mainly using amine solutions that remove the hydrogen sulfide.<ref>NaturalGas.Org. (September 8, 2015). ''Processing Natural Gas'' [Online]. Available: http://naturalgas.org/naturalgas/processing-ng/</ref> | ||
==Uses== | ==Uses== | ||
Hydrogen sulfide is used primarily to produce sulfuric acid and sulfur. | Hydrogen sulfide is used primarily to produce sulfuric acid and sulfur. It is also used to create a variety of inorganic sulfides used to create pesticides, leather, dyes, and pharmaceuticals. Hydrogen sulfide is used to produce [[heavy water]] for [[nuclear power plant]]s (like [[CANDU reactor]]s specifically). Hydrogen sulfide can also be used in agriculture as a disinfectant. It is also widely used in chemical analysis.<ref>CFC. (September 8, 2015). ''Hydrogen Sulfide'' [Online]. Available: http://www.c-f-c.com/specgas_products/hydrogen-sulfide.htm</ref> | ||
Iron smelters, landfills, food processing plants, and breweries are some examples of industrial sources that produce or use hydrogen sulfide. The gas must be disposed of properly as [[emissions]] of hydrogen sulfide can be dangerous.<ref>Kevin Lee. (September 8, 2015). ''Uses for Hydrogen Sulfide'' [Online]. Available: http://www.ehow.com/about_5515916_uses-hydrogen-sulfide.html</ref> | Iron smelters, landfills, food processing plants, and breweries are some examples of industrial sources that produce or use hydrogen sulfide. The gas must be disposed of properly as [[emissions]] of hydrogen sulfide can be dangerous.<ref>Kevin Lee. (September 8, 2015). ''Uses for Hydrogen Sulfide'' [Online]. Available: http://www.ehow.com/about_5515916_uses-hydrogen-sulfide.html</ref> | ||
| Line 29: | Line 30: | ||
The health effects of inhalation of hydrogen sulfide depend on how much a person is exposed to. Hydrogen sulfide is dangerous even at relatively small concentrations as it acts as both an irritant and asphyxiant. At relatively low concentrations, hydrogen sulfide irritates the eyes, nose, and throat. These effects are especially dangerous for children and people with reduced lung function.<ref name=pdf/> | The health effects of inhalation of hydrogen sulfide depend on how much a person is exposed to. Hydrogen sulfide is dangerous even at relatively small concentrations as it acts as both an irritant and asphyxiant. At relatively low concentrations, hydrogen sulfide irritates the eyes, nose, and throat. These effects are especially dangerous for children and people with reduced lung function.<ref name=pdf/> | ||
At certain concentrations, exposure to hydrogen sulfide can result in unconsciousness in | At certain concentrations, exposure to hydrogen sulfide can result in unconsciousness in a couple breaths or nearly instant death. In concentrations as low as 500-700 [[ppm]], hydrogen sulfide can result in death within an hour. At concentrations of only 1000-2000 ppm, hydrogen sulfide can cause near instant death.<ref name=osha/> Health effects of exposure include headaches, memory loss, and problems with the cardiovascular system.<ref name=osha>OSHA. (September 8, 2015). ''Hydrogen Sulfide'' [Online]. Available: https://www.osha.gov/SLTC/hydrogensulfide/hazards.html</ref> Repeated exposure to hydrogen sulfide can result in eye inflammation, fatigue, irritability, insomnia, and weight loss.<ref name=pdf/> | ||
==For Further Reading== | |||
*[[Gas]] | |||
*[[Natural gas]] | |||
*[[Petroleum]] | |||
*[[Carbon monoxide]] | |||
*[[Chemical]] | |||
*Or explore a [[Special:Random|random page]] | |||
==References== | ==References== | ||
{{reflist}} | {{reflist}}[[Category:Uploaded]] | ||
Revision as of 03:38, 6 October 2018
Hydrogen sulfide is a chemical with the formula H2S. Hydrogen sulfide is a dangerous, colourless gas that occurs naturally in many petroleum products. Specifically, it often makes up significant amounts of raw natural gas. It has a distinct "rotten egg" smell at low concentration levels.[2] Prolonged exposure will result in olfactory fatigue, which is the loss of the ability to smell it. At high concentrations of hydrogen sulfide, the ability to smell it is lost instantly.[3] In addition to being harmful to human health as an asphyxiant, hydrogen sulfide is both flammable and explosive.
Hydrogen sulfide exists in natural deposits or can be produced by human activities. For example, hydrogen sulfide occurs naturally in crude oil, natural gas, and in hot springs. Bacterial breakdown of human and animal waste also produced hydrogen sulfide. Humans release hydrogen sulfide through petroleum drilling and refining, wastewater treatment, and paper mills.[2]
Some additional properties of hydrogen sulfide are outlined in the table below.
| Chemical formula | H2S |
| Molar mass | 34.0809 grams/mole |
| Melting point | -82oC[4] |
| Boiling point | -60oC[4] |
The hydrogen sulfide content of natural gas is one of the main safety concerns associated with the use of natural gas. Natural gas with especially high levels of hydrogen sulfide is referred to as sour gas. Some of this sulfur can be removed through a "sweetening" process, called the "amine process" or more commonly the "Girdler process". It is used in most gas sweetening operations and is done mainly using amine solutions that remove the hydrogen sulfide.[5]
Uses
Hydrogen sulfide is used primarily to produce sulfuric acid and sulfur. It is also used to create a variety of inorganic sulfides used to create pesticides, leather, dyes, and pharmaceuticals. Hydrogen sulfide is used to produce heavy water for nuclear power plants (like CANDU reactors specifically). Hydrogen sulfide can also be used in agriculture as a disinfectant. It is also widely used in chemical analysis.[6]
Iron smelters, landfills, food processing plants, and breweries are some examples of industrial sources that produce or use hydrogen sulfide. The gas must be disposed of properly as emissions of hydrogen sulfide can be dangerous.[7]
Health Effects
The health effects of inhalation of hydrogen sulfide depend on how much a person is exposed to. Hydrogen sulfide is dangerous even at relatively small concentrations as it acts as both an irritant and asphyxiant. At relatively low concentrations, hydrogen sulfide irritates the eyes, nose, and throat. These effects are especially dangerous for children and people with reduced lung function.[2]
At certain concentrations, exposure to hydrogen sulfide can result in unconsciousness in a couple breaths or nearly instant death. In concentrations as low as 500-700 ppm, hydrogen sulfide can result in death within an hour. At concentrations of only 1000-2000 ppm, hydrogen sulfide can cause near instant death.[8] Health effects of exposure include headaches, memory loss, and problems with the cardiovascular system.[8] Repeated exposure to hydrogen sulfide can result in eye inflammation, fatigue, irritability, insomnia, and weight loss.[2]
For Further Reading
- Gas
- Natural gas
- Petroleum
- Carbon monoxide
- Chemical
- Or explore a random page
References
- ↑ Wikimedia Commons. (September 8, 2015). Hydrogen Sulfide 3D Balls [Online]. Available: https://upload.wikimedia.org/wikipedia/commons/thumb/3/34/Hydrogen-sulfide-3D-balls.png/1024px-Hydrogen-sulfide-3D-balls.png
- ↑ 2.0 2.1 2.2 2.3 OSHA Fact Sheet. (September 8, 2015). Hydrogen Sulfide [Online]. Available: https://www.osha.gov/OshDoc/data_Hurricane_Facts/hydrogen_sulfide_fact.pdf
- ↑ OSHA QuickCard. (Oct 7, 2018). Hydrogen Sulfide [Online]. Available: https://www.osha.gov/Publications/hydrogen_sulfide.html
- ↑ 4.0 4.1 PubChem. (September 8, 2015). Hydrogen Sulfide [Online]. Available: http://pubchem.ncbi.nlm.nih.gov/compound/hydrogen_sulfide
- ↑ NaturalGas.Org. (September 8, 2015). Processing Natural Gas [Online]. Available: http://naturalgas.org/naturalgas/processing-ng/
- ↑ CFC. (September 8, 2015). Hydrogen Sulfide [Online]. Available: http://www.c-f-c.com/specgas_products/hydrogen-sulfide.htm
- ↑ Kevin Lee. (September 8, 2015). Uses for Hydrogen Sulfide [Online]. Available: http://www.ehow.com/about_5515916_uses-hydrogen-sulfide.html
- ↑ 8.0 8.1 OSHA. (September 8, 2015). Hydrogen Sulfide [Online]. Available: https://www.osha.gov/SLTC/hydrogensulfide/hazards.html