Nuclear chain reaction: Difference between revisions
No edit summary |
m (1 revision imported) |
||
| (8 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2021-10-29]] | ||
[[Category:Translated to French]] | |||
[[fr:Réaction nucléaire en chaîne]] | |||
[[Category:Translated to Spanish]] | |||
[[es:Reacción nuclear en cadena]] | |||
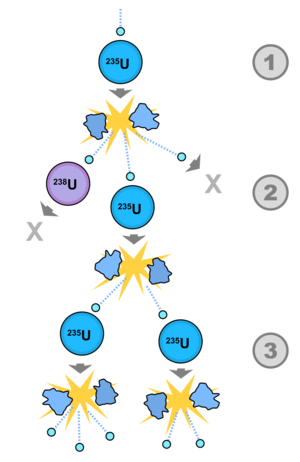
[[File:Fission_chain_reaction.svg.png|thumb|Figure 1. A neutron strikes a <sup>235</sup>U nucleus and causes a fission event. This releases more neutrons. Unlike in the figure, on average one new fission event happens as a result of these released neutrons.]] | |||
<onlyinclude>A '''nuclear chain reaction''' occurs when the output of one [[nuclear reaction]] causes more nuclear reactions to occur. These chain reactions are almost always a series of [[nuclear fission|fission]] events, which give off excess [[neutron]]s. It is these excess neutrons that can go on to cause more fission events to occur, hence the name '''chain reaction'''. Nuclear chain reactions are essential to the operation of nuclear power plants.</onlyinclude> | |||
[[ | Chemical reactions involve different chemical species recombining. Nuclear reactions involve different flavours of nuclei (called [[nuclear species]]) interacting. Many [[chemical reaction]]s are also chain reactions, with many similarities to nuclear chain reactions. These similarities include: | ||
* That the reactions are sustained when chemical or nuclear species available to react. The chain reaction stops when the species are removed or are used up. | |||
* That the chain reactions are controlled (starting, speeding up, slowing down and stopping) by adding or removing chemical or nuclear species in that chain. | |||
* [[Energy]] is often released as the reactions occur. | |||
* Released energy is often output as [[thermal energy]], becoming [[heat]] that can be harnessed by [[heat engine]]s to do useful [[work]] like make [[electricity]]. | |||
While these similarities exist, there are some important differences as well. Nuclear reactions release roughly one '''million''' times as much energy as chemical reactions. This means that chemical chain reactions occur much more easily than nuclear reactions. For example, fire is a chemical chain reaction. Nuclear chain reactions require careful engineering and as far as we know, a natural nuclear chain reaction has only [[Oklo natural nuclear reactor|occurred once.]]<ref> As far as we know! Although it seems unlikely to have occurred more than once.</ref> Nuclear chain reactions require an abundance of careful planning. When they do occur, there is substantially more energy available, leading to nuclear having a much higher [[energy density]] for its fuel. | |||
< | In order to sustain a nuclear chain reaction, every fission event must lead to exactly one more fission event. The most convenient nuclear species to use for nuclear chain reactions is a [[fissile]] [[isotope]] of uranium, <sup>235</sup>U. When <sup>235</sup>U undergoes fission, it gives off, on average, ~2.5 neutrons per fission event. Careful engineering must go into having those neutrons go on to create more fission events. Contrary to what one may expect, difficulties arise in getting enough neutrons to go on and make a sustained nuclear reaction, rather than having too many nuclear reactions. If every fission event leads to ''exactly'' one more fission event, the nuclear chain reaction is said to be '''critical'''. Figure 2 shows a simplification of the fission chain reaction. | ||
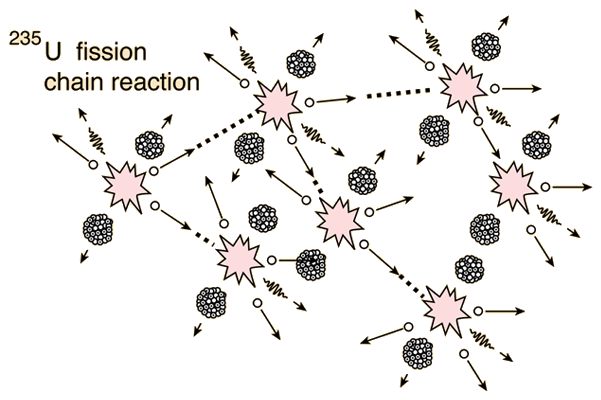
[[File:u235fission.gif|600px|framed|center|Figure 2. A nuclear fission chain reaction of uranium-235 atoms.<ref name=hyp>HyperPhysics. (May 27, 2015). ''Uranium-235 Chain Reaction'' [Online]. Available: http://hyperphysics.phy-astr.gsu.edu/hbase/nucene/u235chn.html</ref> In a real nuclear reactor, most of the released neutrons are lost, rather than leading to another fission event.]] | |||
The video below has a member of the [[About us|Energy Education team]] explaining nuclear chain reactions: | |||
<html><iframe width="560" height="315" src="https://www.youtube.com/embed/MRxbunkSGZU" title="YouTube video player" frameborder="0" allow="accelerometer; autoplay; clipboard-write; encrypted-media; gyroscope; picture-in-picture" allowfullscreen></iframe> </html> | |||
==For Further Reading== | |||
*[[Moderator]] | |||
*[[Isotope]] | |||
*[[Nuclear power plant]] | |||
*[[Nuclear species]] | |||
*[[Strong force]] | |||
*Or explore a [[Special:Random|random page]] | |||
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
[[Category:Uploaded]] | [[Category:Uploaded]] | ||
Latest revision as of 20:21, 20 December 2021
A nuclear chain reaction occurs when the output of one nuclear reaction causes more nuclear reactions to occur. These chain reactions are almost always a series of fission events, which give off excess neutrons. It is these excess neutrons that can go on to cause more fission events to occur, hence the name chain reaction. Nuclear chain reactions are essential to the operation of nuclear power plants.
Chemical reactions involve different chemical species recombining. Nuclear reactions involve different flavours of nuclei (called nuclear species) interacting. Many chemical reactions are also chain reactions, with many similarities to nuclear chain reactions. These similarities include:
- That the reactions are sustained when chemical or nuclear species available to react. The chain reaction stops when the species are removed or are used up.
- That the chain reactions are controlled (starting, speeding up, slowing down and stopping) by adding or removing chemical or nuclear species in that chain.
- Energy is often released as the reactions occur.
- Released energy is often output as thermal energy, becoming heat that can be harnessed by heat engines to do useful work like make electricity.
While these similarities exist, there are some important differences as well. Nuclear reactions release roughly one million times as much energy as chemical reactions. This means that chemical chain reactions occur much more easily than nuclear reactions. For example, fire is a chemical chain reaction. Nuclear chain reactions require careful engineering and as far as we know, a natural nuclear chain reaction has only occurred once.[1] Nuclear chain reactions require an abundance of careful planning. When they do occur, there is substantially more energy available, leading to nuclear having a much higher energy density for its fuel.
In order to sustain a nuclear chain reaction, every fission event must lead to exactly one more fission event. The most convenient nuclear species to use for nuclear chain reactions is a fissile isotope of uranium, 235U. When 235U undergoes fission, it gives off, on average, ~2.5 neutrons per fission event. Careful engineering must go into having those neutrons go on to create more fission events. Contrary to what one may expect, difficulties arise in getting enough neutrons to go on and make a sustained nuclear reaction, rather than having too many nuclear reactions. If every fission event leads to exactly one more fission event, the nuclear chain reaction is said to be critical. Figure 2 shows a simplification of the fission chain reaction.
The video below has a member of the Energy Education team explaining nuclear chain reactions:
For Further Reading
References
- ↑ As far as we know! Although it seems unlikely to have occurred more than once.
- ↑ HyperPhysics. (May 27, 2015). Uranium-235 Chain Reaction [Online]. Available: http://hyperphysics.phy-astr.gsu.edu/hbase/nucene/u235chn.html