Thermal conduction: Difference between revisions
m (1 revision imported) |
energy>Jmdonev No edit summary |
||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2020-02-29]] | ||
<onlyinclude>'''Thermal conduction''' is the | [[File:whatisheatco.jpg|400px|thumb|right|Figure 1. Heat transfers from a hot surface to a cold surface through thermal conduction.<ref> Retrieved from https://phys.org/news/2014-12-what-is-heat-conduction.html</ref>]] | ||
<onlyinclude>'''Thermal conduction''' is the diffusion of [[thermal energy]] (heat) within one material or between materials in contact.</onlyinclude> The higher temperature object has molecules with more kinetic energy; collisions between molecules distributions this kinetic energy until an object has the same thermal energy throughout. ''Conduction is the main mode of heat transfer between or inside solid materials''. Conduction allows gas and electric stoves heat up pans. Buildings lose much of [[energy loss|their heat]] by conduction, the pockets of air in [[insulation]] prevents this loss by stopping conduction.<ref>Keeping the heat in, Chapter 2, NR CAN available https://www.nrcan.gc.ca/energy-efficiency/energy-efficiency-homes/make-your-home-more-energy-efficient/keeping-heat/keeping-heat-chapter-2-how-your-house-works/15630 accessed January 19th, 2020.</ref> | |||
==Thermal Conduction== | ==Thermal Conduction== | ||
A region with greater thermal energy (heat) corresponds with greater molecular agitation. Thus when a hot object touches a cooler surface, the highly agitated molecules from the hot object bump the calm molecules of the cooler surface, transferring the [[kinetic energy]] and causing | A region with greater thermal energy (heat) corresponds with greater molecular agitation. Thus when a hot object touches a cooler surface, the highly agitated molecules from the hot object bump the calm molecules of the cooler surface, transferring the microscopic [[kinetic energy]] and causing the colder part or object to heat up. | ||
Mathematically, thermal conduction works just like diffusion. As temperature difference goes up, the distance traveled gets shorter or the area goes up thermal conduction increases: | |||
<center><big>Thermal conduction (power)<math>= \frac{\kappa A \Delta T}{\ell}</math></big></center> | |||
Where: | |||
* Thermal conduction (power) is the heat per unit time transferred some distance <math>\ell</math> between the two temperatures. | |||
* <math>\kappa</math> is the [[thermal conductivity]] of the material | |||
* <math>A</math> is the [[cross-sectional area]] of the object | |||
* <math>\Delta T </math> is the difference in temperature from one side to the other. | |||
* <math>\ell</math> is the length of the path the heat has to be transferred. | |||
Conduction is the main mode of heat transfer for solid materials because the strong inter-molecular forces allow the vibrations of particles to be easily transmitted, in comparison to liquids and gases. Liquids have weaker inter-molecular forces and more space between the particles, which makes the vibrations of particles harder to transmit. Gases have even more space, and therefore infrequent particle collisions. This makes liquids and gases poor conductors of heat. | |||
Good thermal conduction is essential for [[heat exchanger]]s, which allow heat to be exchanged between two fluids without mixing them. For example, an [[external heat engine]], (such as a [[coal fired power plant]] or a [[nuclear power plant]]) generates heat, which must be transferred to a [[working fluid]] in order to generate electricity. This produced heat must be transferred by thermal conduction. | |||
==For Further Reading== | |||
*[[Heat exchanger]] | |||
*[[Convection]] | |||
*[[Refrigerator]] | |||
*[[Boiler]] | |||
*[[Heat]] | |||
*Or explore a [[Special:Random|random page]] | |||
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
[[Category:Uploaded]] | [[Category:Uploaded]] | ||
Revision as of 08:35, 20 February 2020
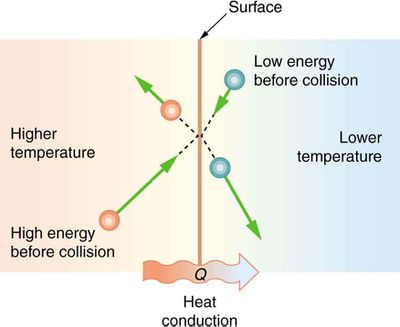
Thermal conduction is the diffusion of thermal energy (heat) within one material or between materials in contact. The higher temperature object has molecules with more kinetic energy; collisions between molecules distributions this kinetic energy until an object has the same thermal energy throughout. Conduction is the main mode of heat transfer between or inside solid materials. Conduction allows gas and electric stoves heat up pans. Buildings lose much of their heat by conduction, the pockets of air in insulation prevents this loss by stopping conduction.[2]
Thermal Conduction
A region with greater thermal energy (heat) corresponds with greater molecular agitation. Thus when a hot object touches a cooler surface, the highly agitated molecules from the hot object bump the calm molecules of the cooler surface, transferring the microscopic kinetic energy and causing the colder part or object to heat up. Mathematically, thermal conduction works just like diffusion. As temperature difference goes up, the distance traveled gets shorter or the area goes up thermal conduction increases:
Where:
- Thermal conduction (power) is the heat per unit time transferred some distance between the two temperatures.
- is the thermal conductivity of the material
- is the cross-sectional area of the object
- is the difference in temperature from one side to the other.
- is the length of the path the heat has to be transferred.
Conduction is the main mode of heat transfer for solid materials because the strong inter-molecular forces allow the vibrations of particles to be easily transmitted, in comparison to liquids and gases. Liquids have weaker inter-molecular forces and more space between the particles, which makes the vibrations of particles harder to transmit. Gases have even more space, and therefore infrequent particle collisions. This makes liquids and gases poor conductors of heat.
Good thermal conduction is essential for heat exchangers, which allow heat to be exchanged between two fluids without mixing them. For example, an external heat engine, (such as a coal fired power plant or a nuclear power plant) generates heat, which must be transferred to a working fluid in order to generate electricity. This produced heat must be transferred by thermal conduction.
For Further Reading
- Heat exchanger
- Convection
- Refrigerator
- Boiler
- Heat
- Or explore a random page
References
- ↑ Retrieved from https://phys.org/news/2014-12-what-is-heat-conduction.html
- ↑ Keeping the heat in, Chapter 2, NR CAN available https://www.nrcan.gc.ca/energy-efficiency/energy-efficiency-homes/make-your-home-more-energy-efficient/keeping-heat/keeping-heat-chapter-2-how-your-house-works/15630 accessed January 19th, 2020.

