Thermal efficiency: Difference between revisions
J.williams (talk | contribs) m (1 revision imported) |
No edit summary |
||
| Line 1: | Line 1: | ||
[[category:371 topics]] | [[category:371 topics]] | ||
[[Category:Done 2015- | [[Category:Done 2015-09-06]] | ||
[[category:301 topics]] | [[category:301 topics]] | ||
[[Category:waiting for simulation]] | [[Category:waiting for simulation]] | ||
[[File:Flow Chart thing.png|340px|thumbnail|Figure 1: The amount of work output for a given amount of heat gives a system its thermal efficiency.<ref>This picture was made by the Energy Education team.</ref>]] | [[File:Flow Chart thing.png|340px|thumbnail|Figure 1: The amount of work output for a given amount of heat gives a system its thermal efficiency.<ref>This picture was made by the Energy Education team.</ref>]] | ||
<onlyinclude>The '''thermal efficiency''' of a [[heat engine]] is the amount of useful work an engine can do based on the amount of heat input.</onlyinclude> The thermal efficiency is represented by the symbol <m>\eta</m>, and given by the equation | <onlyinclude>The '''thermal efficiency''' of a [[heat engine]] is the amount of useful [[work]] an engine can do based on the amount of [[heat]] input.</onlyinclude> The thermal efficiency is represented by the symbol <m>\eta</m>, and given by the equation | ||
<center><m>\eta=\frac{W}{Q_H}</m></center> | <center><m>\eta=\frac{W}{Q_H}</m></center> | ||
| Line 23: | Line 23: | ||
==Carnot Efficiency== | ==Carnot Efficiency== | ||
:''[[Carnot efficiency|main article]]'' | :''[[Carnot efficiency|main article]]'' | ||
There is a maximum attainable efficiency of a heat engine which was derived by physicist Sadi Carnot. Following laws of thermodynamics the equation for this turns out to be | There is a maximum attainable efficiency of a heat engine which was derived by physicist Sadi Carnot. Following laws of [[thermodynamics]] the equation for this turns out to be | ||
<center><m>\eta_{max}=1 - \frac{T_L}{T_H}</m></center> | <center><m>\eta_{max}=1 - \frac{T_L}{T_H}</m></center> | ||
| Line 29: | Line 29: | ||
Where | Where | ||
<m>T_L</m> is the temperature of the cold 'sink' | <m>T_L</m> is the [[temperature]] of the cold 'sink' | ||
and | and | ||
<m>T_H</m> is the temperature of the heat reservoir. | <m>T_H</m> is the temperature of the heat reservoir. | ||
This describes the efficiency of an idealized engine, which in reality is impossible to achieve.<ref>Hyperphysics, ''Carnot Cycle'' [Online], Available: http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/carnot.html</ref> From this equation, the lower the sink temperature <m>T_L</m> or the higher the source temperature <m>T_H</m>, the more work is available from the heat engine. The energy for work comes from a decrease in the total energy of the fluid used in the system. Therefore the greater the temperature change, the greater this decrease in the fluid and thus the greater energy available to do work is.<ref>R. A. Hinrichs and M. Kleinbach, "Heat and Work," in ''Energy: Its Use and the Environment'', 4th ed. Toronto, Ont. Canada: Thomson Brooks/Cole, 2006, ch.4, sec.E, pp.115</ref> | This describes the efficiency of an idealized engine, which in reality is impossible to achieve.<ref>Hyperphysics, ''Carnot Cycle'' [Online], Available: http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/carnot.html</ref> From this equation, the lower the sink temperature <m>T_L</m> or the higher the source temperature <m>T_H</m>, the more work is available from the heat engine. The [[energy]] for work comes from a decrease in the total energy of the [[fluid]] used in the [[system]]. Therefore the greater the temperature change, the greater this decrease in the fluid and thus the greater energy available to do work is.<ref>R. A. Hinrichs and M. Kleinbach, "Heat and Work," in ''Energy: Its Use and the Environment'', 4th ed. Toronto, Ont. Canada: Thomson Brooks/Cole, 2006, ch.4, sec.E, pp.115</ref> | ||
Revision as of 23:37, 4 September 2015
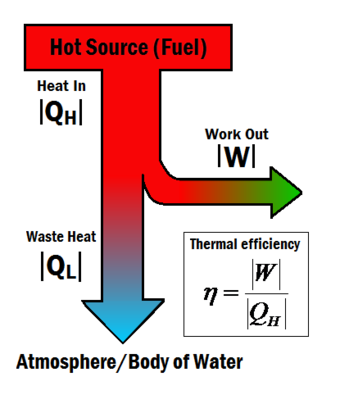
The thermal efficiency of a heat engine is the amount of useful work an engine can do based on the amount of heat input. The thermal efficiency is represented by the symbol , and given by the equation
Where:
is the useful work and
is the total heat energy input from the hot source.[2]
Heat engines often operate at around 30% to 50% efficiency, due to practical limitations. It is impossible for heat engines to achieve 100% thermal efficiency () according to the Second law of thermodynamics. This is impossible because some waste heat is always produced produced in a heat engine, shown in Figure 1 by the
term. Although complete efficiency in a heat engine is impossible, there are many ways to increase a system's overall efficiency.
An Example
If 200 joules of thermal energy as heat is input (), and the engine does 80 J of work (
), then the efficiency is 80J/200J, which is 40% efficient.
This same result can be gained by measuring the waste heat of the engine. For example, if 200 J is put into the engine, and observe 120 J of waste heat, then 80 J of work must have been done, giving 40% efficiency.
Carnot Efficiency
There is a maximum attainable efficiency of a heat engine which was derived by physicist Sadi Carnot. Following laws of thermodynamics the equation for this turns out to be
Where
is the temperature of the cold 'sink'
and
is the temperature of the heat reservoir.
This describes the efficiency of an idealized engine, which in reality is impossible to achieve.[3] From this equation, the lower the sink temperature or the higher the source temperature
, the more work is available from the heat engine. The energy for work comes from a decrease in the total energy of the fluid used in the system. Therefore the greater the temperature change, the greater this decrease in the fluid and thus the greater energy available to do work is.[4]
Click here to learn about how a heat engine works.
For thermal efficiency of car engines, click here.
References
- ↑ This picture was made by the Energy Education team.
- ↑ TPUB Engine Mechanics. (April 4, 2015). Thermal Efficiency [Online]. Available: http://enginemechanics.tpub.com/14075/css/14075_141.htm
- ↑ Hyperphysics, Carnot Cycle [Online], Available: http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/carnot.html
- ↑ R. A. Hinrichs and M. Kleinbach, "Heat and Work," in Energy: Its Use and the Environment, 4th ed. Toronto, Ont. Canada: Thomson Brooks/Cole, 2006, ch.4, sec.E, pp.115

