Valence and core electrons: Difference between revisions
J.williams (talk | contribs) No edit summary |
J.williams (talk | contribs) m (1 revision imported) |
(No difference)
| |
Revision as of 21:31, 26 August 2015
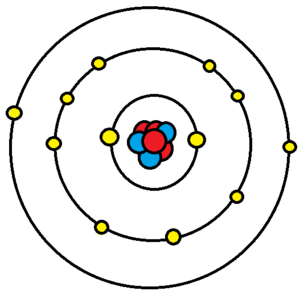
Electrons exist in orbitals around a nucleus. These orbitals and the associated energy needed to remove each of these electrons are set by quantum mechanics. Each of these orbitals serves to create a shell of electrons in the atom. Valence electrons are the electrons orbiting the nucleus in the outermost atomic shell of an atom. Electrons that are closer to the nucleus are in filled orbitals and are called core electrons. Valence electrons are the farthest from the positive charge (the protons) and thus tend to be easier to remove than core electrons; this means that it takes them less energy to move far away from the atom. This difference comes from the electric force being an inverse square law. Removing an electron (any of them) from a neutral atom turns the atom into an ion.
Valence electrons also tend to be the electrons that specifically participate in a chemical bond, like ionic bonds or covalent bonds.
For more information about valence electrons, core electrons and how they related to chemical reactions please see UC Davis's chem wiki.
References
- ↑ This image was created by part of the Energy Education team.

