Alcohol
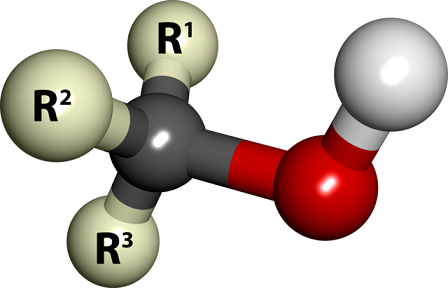
An alcohol is a type of organic compound that is composed of a carbon and hydrogen alkyl chain with one or more hydroxy functional groups attached to a carbon atom of the main hydrocarbon chain. This hydroxyl group is composed of one oxygen and one hydrogen atom bonded together and is represented by . Alcohols are sometimes classified as derivatives of water because the molecules have similar structures. A generic alcohol is generally shown (see figure 1) as being a hydroxyl group attached to one singular carbon atom, with the rest of the potentially longer carbon chain represented only by .[2] Alcohols can also be classified as being primary, secondary, or tertiary depending on where this hydroxyl group is located on the carbon-carbon chain (on the first, second, or third carbon respectively).
Alcohols have several different uses. Methanol and ethanol can be used as alternative fuels in vehicles due to the fact that they burn very cleanly. Some alcohols, specifically ethanol, are used as solvents in products such as perfumes, cosmetics, and plant extracts to keep them "fresh" and act as an alternative solvent for compounds that are insoluble in water. They can also be used as cleaning agents for items like paint brushes.[3] Only ethanol is considered safe for consumption and can be referred to as "drinking alcohol".
Properties
Alcohols are generally colourless and liquid at room temperature. Due to the polarity of their bonds, they are also highly soluble in water. However this solubility tends to decrease as the size and weight of the molecule increase. As well, as their molecular weight increases, their boiling points, densities, and viscosities increase.[4] Many smaller alcohols like ethanol considered volatile[5] which helps them burn.
For more information on alcohols, please visit UC Davis' Chem Wiki
References
- ↑ "Alcohol" Licensed under Public Domain via Wikimedia Commons - http://commons.wikimedia.org/wiki/File:Alcohol.png#mediaviewer/File:Alcohol.png
- ↑ Leroy G. Wade, Jr. (September 3, 2014). Alcohols [Online]. Available: http://www.britannica.com/EBchecked/topic/13366/alcohol [March 2, 2015]
- ↑ At-Bristol Science Center. (2014). Alcohol Uses [Online]. Available: http://www.alcoholandyou.org.uk/facts/uses.html [March 2, 2015]
- ↑ Jim Clark. (2003). An Introduction to Alcohols [Online]. Available: http://www.chemguide.co.uk/organicprops/alcohols/background.html [March 2, 2015]
- ↑ Ethanol [Online]. Available: http://www.npi.gov.au/resource/ethanol-ethyl-alcohol [March 16th, 2015]

