Conduction band
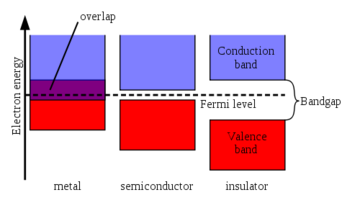
The conduction band is the band of electron orbitals that electrons can jump up into from the valence band when excited. When the electrons are in these orbitals, they have enough energy to move freely in the material. This movement of electrons creates an electric current. The valence band is simply the outermost electron orbital of an atom of any specific material that electrons actually occupy. The energy difference between the highest occupied energy state of the valence band and the lowest unoccupied state of the conduction band is called the band gap and is indicative of the electrical conductivity of a material.[3] A large band gap means that a lot of energy is required to excite valence electrons to the conduction band. Conversely, when the valence band and conduction band overlap as they do in metals, electrons can readily jump between the two bands (see Figure 1) meaning the material is highly conductive.[4]
Insulators are characterized by a large band gap, so a prohibitively large amount of energy is required to move electrons into the conduction band to form a current.[5] Conductors have an overlap between the conduction and valence bands, so the valence electrons in such conductors are essentially free.[4] Semiconductors, on the other hand, have a small band gap that allows for a meaningful fraction of the valence electrons of the material to move into the conduction band given a certain amount of energy. This property gives them a conductivity between conductors and insulators, which is part of the reason why they are ideal for electric circuits as they will not cause a short circuit like a conductor.[2] This band gap also allows semiconductors to convert light into electricity in photovoltaic cells and to emit light as LEDs when made into certain types of diodes. Both these processes rely on the energy absorbed or released by electrons moving between the conduction and valence bands.
For Further Reading
For further information please see the related pages below:
- Semiconductor
- Metal
- Photovoltaic cell
- AC to DC adapter
- Or explore a random page!
References
- ↑ Wikimedia Commons. File:Isolator-metal.svg [Online]. Available: https://commons.wikimedia.org/wiki/File:Isolator-metal.svg
- ↑ 2.0 2.1 UC Davis ChemWiki. (August 14, 2015). Band Theory of Semiconductors [Online]. Available: http://chemwiki.ucdavis.edu/u_Materials/Electronic_Properties/Band_Theory_of_Semiconductors
- ↑ Introduction to Energy Bands [Online]. Available:http://www.doitpoms.ac.uk/tlplib/semiconductors/energy_band_intro.php
- ↑ 4.0 4.1 Hyperphysics. (August 14, 2015). Conductor Energy Bands [Online]. Available:http://hyperphysics.phy-astr.gsu.edu/hbase/solids/band.html#c6
- ↑ Hyperphysics. (August 14, 2015). Insulator Energy Bands [Online]. Available: http://hyperphysics.phy-astr.gsu.edu/hbase/solids/band.html#c4

