Carbohydrate: Difference between revisions
J.williams (talk | contribs) m (1 revision imported) |
energy>Jmdonev No edit summary |
||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2021-01-31]] | ||
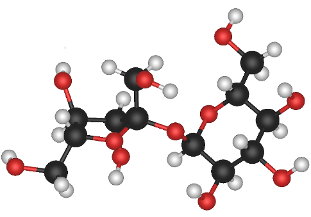
[[File:Sucrose_molecule_3d_model_resized.png|frame|right|Figure 1. A model of Sucrose, one of the simplest and most commonly found carbohydrates.<ref> Akane700. (2006).''Sucrose Molecule 3D Model''[Online]. Available:http://simple.wikipedia.org/wiki/Sucrose#/media/File:Sucrose_molecule_3d_model.png</ref> The black spheres represent carbon, the red oxygen and the white are hydrogen.]] | [[File:Sucrose_molecule_3d_model_resized.png|frame|right|Figure 1. A model of Sucrose, one of the simplest and most commonly found carbohydrates.<ref> Akane700. (2006).''Sucrose Molecule 3D Model''[Online]. Available:http://simple.wikipedia.org/wiki/Sucrose#/media/File:Sucrose_molecule_3d_model.png</ref> The black spheres represent carbon, the red oxygen and the white are hydrogen.]] | ||
<onlyinclude>'''Carbohydrates''' are [[organic molecule]]s made of [[carbon]], [[oxygen]], and [[hydrogen]]. Carbohydrates are found in all living [[organism]]s. They undergo metabolic reactions that provide [[energy]] to keep bodies functioning to sustain life.<ref>“Carbohydrates.” August 10, 2020. [Online]. Available: https://chem.libretexts.org/@go/page/377.</ref><ref name="RE1"> ''Chemistry,'' Rice University, 2015. [Online]. Available: https://web.ung.edu/media/Chemistry2/Chemistry-LR.pdf</ref></onlyinclude> The smaller carbohydrates are generally referred to as “sugars". Carbohydrates are initially made from [[photosynthesis]], which is the process plants use to take in [[carbon dioxide]], and is one of the most important ways of getting carbon dioxide out of the [[atmosphere]] (although not the only way). The name “carbohydrate” comes from the formula of the molecules, which can be described by the general formula C<sub>n</sub>(H<sub>2</sub>O)<sub>n</sub>. This formula shows that they are in a sense “carbon and water” or “hydrates of carbon.” In many cases, the number of carbon atoms and water molecules will have the same value (n), but they can be different.<ref name="RE1"/> The [[chemical reaction]] to form carbohydrates takes some number of [[carbon dioxide]] [[molecule]]s, adds the same number of water molecules, and makes a sugar with a chain of carbon atoms:<ref name=libre>“Carbohydrates.” September 12, 2020. [Online]. Available: https://chem.libretexts.org/@go/page/5628.</ref> | |||
<center> | |||
<math> n CO_2 + n H_2O + Energy \rightarrow C_nH_{2n}O_n + n O_2</math> | |||
</center> | |||
Carbohydrates usually have a ratio of two hydrogen for one oxygen (just like [[water]]) along with the same number of oxygen [[atom]]s and carbon atoms.<ref name=libre/> Many molecules that give [[energy]] to living organisms get that energy from some sort of carbohydrate like sugars, starch, or [[cellulose]]. Overall, carbohydrates store energy, provide structural support, and have a variety of other biological functions.<ref name="RE1"/> | |||
For more information about carbohydrates please see UC Davis's [https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Carbohydrates chem wiki]. | |||
For more information about carbohydrates please see UC Davis's [ | |||
==For Further Reading== | |||
*[[Chemical bond]] | |||
*[[Chemical reaction]] | |||
*[[Chemical energy]] | |||
*Or explore a [[Special:Random|random page]] | |||
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
[[Category:Uploaded]] | [[Category:Uploaded]] | ||
Revision as of 22:34, 25 May 2021
Carbohydrates are organic molecules made of carbon, oxygen, and hydrogen. Carbohydrates are found in all living organisms. They undergo metabolic reactions that provide energy to keep bodies functioning to sustain life.[2][3] The smaller carbohydrates are generally referred to as “sugars". Carbohydrates are initially made from photosynthesis, which is the process plants use to take in carbon dioxide, and is one of the most important ways of getting carbon dioxide out of the atmosphere (although not the only way). The name “carbohydrate” comes from the formula of the molecules, which can be described by the general formula Cn(H2O)n. This formula shows that they are in a sense “carbon and water” or “hydrates of carbon.” In many cases, the number of carbon atoms and water molecules will have the same value (n), but they can be different.[3] The chemical reaction to form carbohydrates takes some number of carbon dioxide molecules, adds the same number of water molecules, and makes a sugar with a chain of carbon atoms:[4]
Carbohydrates usually have a ratio of two hydrogen for one oxygen (just like water) along with the same number of oxygen atoms and carbon atoms.[4] Many molecules that give energy to living organisms get that energy from some sort of carbohydrate like sugars, starch, or cellulose. Overall, carbohydrates store energy, provide structural support, and have a variety of other biological functions.[3]
For more information about carbohydrates please see UC Davis's chem wiki.
For Further Reading
- Chemical bond
- Chemical reaction
- Chemical energy
- Or explore a random page
References
- ↑ Akane700. (2006).Sucrose Molecule 3D Model[Online]. Available:http://simple.wikipedia.org/wiki/Sucrose#/media/File:Sucrose_molecule_3d_model.png
- ↑ “Carbohydrates.” August 10, 2020. [Online]. Available: https://chem.libretexts.org/@go/page/377.
- ↑ 3.0 3.1 3.2 Chemistry, Rice University, 2015. [Online]. Available: https://web.ung.edu/media/Chemistry2/Chemistry-LR.pdf
- ↑ 4.0 4.1 “Carbohydrates.” September 12, 2020. [Online]. Available: https://chem.libretexts.org/@go/page/5628.

