Hydrocarbon
The term hydrocarbon refers to the most basic type of organic molecules. They are comprised of only 2 elements: hydrogen and carbon, hence the name hydrocarbons.[1] In general, hydrocarbon molecules are structured with one or more carbon atoms forming a central structure that is surrounded by hydrogen atoms. There are four main types of hydrocarbons: Alkanes, Alkenes, Alkynes, and Aromatic hydrocarbons.[2]
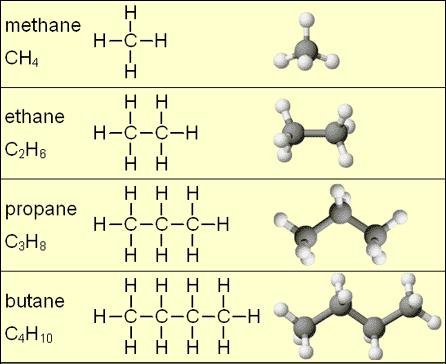
The simplest hydrocarbons are called alkanes. Alkanes are characterized by a simple carbon chain where every carbon atom is bonded to 2 hydrogen atoms and 2 carbon atoms, except the carbons at the end of the chain which are bonded to a third hydrogen, instead of a second carbon. An example of alkanes is seen in figure 1, which shows that all alkanes are made up of single bonds. Hydrocarbon geometry can become very complex as molecules get larger and potentially contain branches and cyclic “loops” of carbon atoms.
Sometimes, elements other than hydrogen and carbon can replace a hydrogen or carbon in a hydrocarbon molecule. For example, a hydrogen may be replaced with a bromine or hydroxide ion. There is a vast number of possible impure hydrocarbons that contain one or more different elements. In general, a hydrocarbon that contains elements other than hydrogen and carbon is not a true hydrocarbon and is called a hydrocarbon derivative.[1]
Hydrocarbons and their derivatives are the main constituents of fossil fuels and release energy when burning with oxygen. For a more complete description of this process, see the Hydrocarbon combustion page.
In addition to fuels, hydrocarbons are used in many other applications. Certain hydrocarbons can be found in lubricating oils, greases, solvents, fuels, wax, asphalts, cosmetics, and plastics.[4] These hydrocarbons are also products of fractional distillation. Although hydrocarbons are primarily consumed in fuels, non-fuel applications of hydrocarbons are of great importance to society and the economy.
To learn more about Hydrocarbons, check out the UC Davis Chem Wiki.
For Further reading
Check out the specific types of hydrocarbons:
References
- ↑ 1.0 1.1 C. Nave. "Hydrocarbons." Internet: http://hyperphysics.phy-astr.gsu.edu/hbase/organic/hydrocarbon.html, [October 25, 2013].
- ↑ "Hydrocarbons - Chemistry LibreTexts", Chem.libretexts.org, 2018. [Online]. Available: https://chem.libretexts.org/Core/Organic_Chemistry/Hydrocarbons. [Accessed: 07- Jun- 2018].
- ↑ D. Darling. "Alkanes." Internet: http://www.daviddarling.info/encyclopedia/A/alkane.html, [October 26, 2013]
- ↑ R.D. Botts, D.M. Carson, and D.Coglon. "Petroleum in our live" in Our petroleum challenge, 8th ed. Calgary:Canadian Center for Energy Development, 2013, pp. 7-15.

