Isothermal: Difference between revisions
m (1 revision imported) |
No edit summary |
||
| Line 1: | Line 1: | ||
[[Category:Done 2018- | [[Category:Done 2018-06-15]] | ||
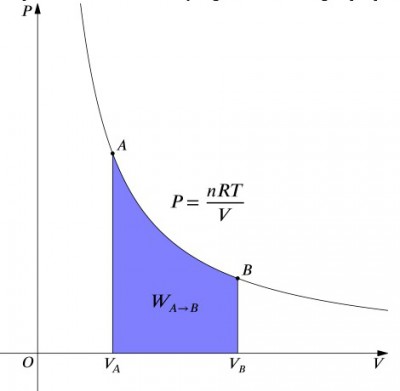
[[File:isothermal PV cycle .jpg|thumb|400px|right| The [[ | [[File:isothermal PV cycle .jpg|thumb|400px|right| The [[Pressure volume diagram]] of an isothermal process.]] | ||
<onlyinclude>'''Isothermal''' refers to a process in which a [[system]] changes—whether it be the [[pressure]], [[volume]] and/or contents—without the [[temperature]] changing.</onlyinclude> From the point of view of the [[first law of thermodynamics]], this means that the [[internal energy]] of the system is unchanged, since temperature is a measure of the average [[kinetic energy]] of [[molecule]]s within the system.<ref name=gould>H. Gould and J. Tobochnik, "Temperature," in ''Statistical and Thermal Physics'', 1st ed., Princeton, NJ: Princeton University Press, 2010, ch.2, sec.4, pp. 35-38</ref> This then looks like: | <onlyinclude>'''Isothermal''' refers to a process in which a [[system]] changes—whether it be the [[pressure]], [[volume]] and/or contents—without the [[temperature]] changing.</onlyinclude> From the point of view of the [[first law of thermodynamics]], this means that the [[internal energy]] of the system is unchanged, since temperature is a measure of the average [[kinetic energy]] of [[molecule]]s within the system.<ref name=gould>H. Gould and J. Tobochnik, "Temperature," in ''Statistical and Thermal Physics'', 1st ed., Princeton, NJ: Princeton University Press, 2010, ch.2, sec.4, pp. 35-38</ref> This then looks like: | ||
| Line 16: | Line 15: | ||
*<math>W</math> is [[work]] | *<math>W</math> is [[work]] | ||
What these equations mean is that the work input to a system must be ''exactly'' balanced by a heat output, and vice versa. If an insulated container containing [[air]] is compressed (decreasing its volume, positive <math>W</math> value), then heat must be removed from the system in accordance (negative <math>Q</math> value). In contrast, if a container is allowed to expand (negative <math>W</math>), then heat must be added to the system in order to keep the temperature constant. | What these equations mean is that the work input to a system must be ''exactly'' balanced by a heat output, and vice versa. If an insulated container containing [[air]] is compressed (decreasing its volume, positive <math>W</math> value), then heat must be removed from the system in accordance (negative <math>Q</math> value). In contrast, if a container is allowed to expand (negative <math>W</math>), then heat must be added to the system in order to keep the temperature constant. To calculate work, an integration must be done to the formula <math>W = -∫pdV</math>. This can also be thought of as calculating the area under the curve. However, due to the shape of the curve, it's not as simple of a calculation—in comparison to an [[isobar|isobaric]] process, for example. The formula below is the integrated equation, and will calculate the work done for any isothermal process: | ||
<center><math>W = -nRTln\frac{V_{f}}{V_{i}} = -p_{i}V_{i}ln\frac{V_{f}}{V_{i}} = -p_{f}V_{f}ln\frac{V_{f}}{V_{i}}</math> <ref>R. Knight, Physics for scientists and engineers. San Fransisco: Pearson Addison Wesley, 2008, p. 512.</ref></center> | |||
where: | |||
* <math>n</math> is the number of moles | |||
*<math>R</math> is the [[ideal gas constant]] | |||
*<math>p_{i}</math> is the initial pressure | |||
*<math>V_{f}</math> is the final volume | |||
The [[Carnot efficiency]] explaining the maximum [[thermal efficiency]] of a [[heat engine]] is derived by using isothermal processes, in which a thermodynamic cycle is completed with the use of 2 isothermal and 2 [[adiabatic]] processes.<ref name=Knight>R. D. Knight, "The Limits of Efficiency" in ''Physics for Scientists and Engineers: A Strategic Approach,'' 3nd ed. San Francisco, U.S.A.: Pearson Addison-Wesley, 2008, ch.19, sec.5, pp. 540-542</ref> [[Phase change]]s are an example of isothermal processes, since the temperature remains constant until the phase change is complete. | The [[Carnot efficiency]] explaining the maximum [[thermal efficiency]] of a [[heat engine]] is derived by using isothermal processes, in which a thermodynamic cycle is completed with the use of 2 isothermal and 2 [[adiabatic]] processes.<ref name=Knight>R. D. Knight, "The Limits of Efficiency" in ''Physics for Scientists and Engineers: A Strategic Approach,'' 3nd ed. San Francisco, U.S.A.: Pearson Addison-Wesley, 2008, ch.19, sec.5, pp. 540-542</ref> [[Phase change]]s are an example of isothermal processes, since the temperature remains constant until the phase change is complete. | ||
Revision as of 01:19, 9 June 2018
Isothermal refers to a process in which a system changes—whether it be the pressure, volume and/or contents—without the temperature changing. From the point of view of the first law of thermodynamics, this means that the internal energy of the system is unchanged, since temperature is a measure of the average kinetic energy of molecules within the system.[1] This then looks like:
and consequently,
where:
What these equations mean is that the work input to a system must be exactly balanced by a heat output, and vice versa. If an insulated container containing air is compressed (decreasing its volume, positive value), then heat must be removed from the system in accordance (negative value). In contrast, if a container is allowed to expand (negative ), then heat must be added to the system in order to keep the temperature constant. To calculate work, an integration must be done to the formula . This can also be thought of as calculating the area under the curve. However, due to the shape of the curve, it's not as simple of a calculation—in comparison to an isobaric process, for example. The formula below is the integrated equation, and will calculate the work done for any isothermal process:
where:
- is the number of moles
- is the ideal gas constant
- is the initial pressure
- is the final volume
The Carnot efficiency explaining the maximum thermal efficiency of a heat engine is derived by using isothermal processes, in which a thermodynamic cycle is completed with the use of 2 isothermal and 2 adiabatic processes.[3] Phase changes are an example of isothermal processes, since the temperature remains constant until the phase change is complete.
The following video from UC Berkley's chemistry department explains the idea of an isothermal process with visuals.
References
- ↑ H. Gould and J. Tobochnik, "Temperature," in Statistical and Thermal Physics, 1st ed., Princeton, NJ: Princeton University Press, 2010, ch.2, sec.4, pp. 35-38
- ↑ R. Knight, Physics for scientists and engineers. San Fransisco: Pearson Addison Wesley, 2008, p. 512.
- ↑ R. D. Knight, "The Limits of Efficiency" in Physics for Scientists and Engineers: A Strategic Approach, 3nd ed. San Francisco, U.S.A.: Pearson Addison-Wesley, 2008, ch.19, sec.5, pp. 540-542

