Law of conservation of charge: Difference between revisions
J.williams (talk | contribs) m (1 revision imported) |
No edit summary |
||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2018-07-20]] | ||
<onlyinclude>'''Law of conservation of charge''' says that the net [[charge]] of an isolated system will always remain constant.</onlyinclude><ref name=col>J. Wilson and A. Buffa, "Electric Charge" in ''College Physics'', 5th ed., Upper Saddle River, NJ: Pearson, 2003, ch.15, sec.1, pp.515-516</ref> This means that any system that is not exchanging [[mass]] or [[energy]] with its surroundings will never have a different total charge at any two times. For example if two objects in an isolated system have a net charge of zero, and one object exchanges one million electrons to the other | <onlyinclude>'''Law of conservation of charge''' says that the net [[charge]] of an [[system and surrounding|isolated system]] will always remain constant.</onlyinclude><ref name=col>J. Wilson and A. Buffa, "Electric Charge" in ''College Physics'', 5th ed., Upper Saddle River, NJ: Pearson, 2003, ch.15, sec.1, pp.515-516</ref> This means that any system that is not exchanging [[mass]] or [[energy]] with its surroundings will never have a different total charge at any two times. For example, if two objects in an isolated system have a net charge of zero, and one object exchanges one million electrons to the other, the object with the excess electrons will be negatively charged and the object with the reduced number of electrons will have a positive charge of the ''same magnitude''. The total charge of the system has not and will never change.<ref name=col/> | ||
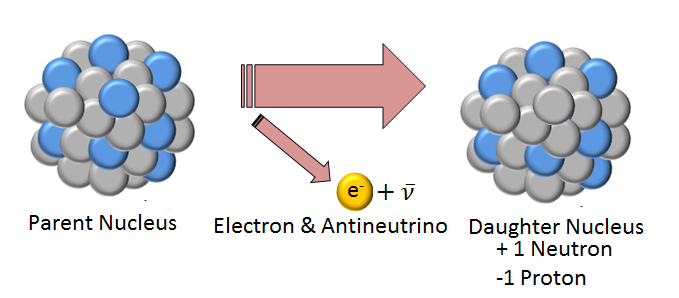
This concept is important for all [[nuclear energy|nuclear]] | This concept is important for all [[nuclear energy|nuclear]] reactions—[[alpha decay]], [[beta decay]], [[gamma decay]], etc.— because it allows scientists to predict the composition of the final product in the reaction, shown in Figure 1.<ref>J. Wilson and A. Buffa, "Radioactivity" in ''College Physics'', 5th ed., Upper Saddle River, NJ: Pearson, 2003, ch.29, sec.2, pp.933</ref> | ||
Charged particles are allowed to be created or destroyed, as long as the net charge before and after the creation/destruction stays the same. Therefore this must happen with oppositely charged pairs of matter and anti-matter.<ref name=col/> | Charged particles are allowed to be created or destroyed, as long as the net charge before and after the creation/destruction stays the same. Therefore this must happen with oppositely charged pairs of matter and anti-matter.<ref name=col/> | ||
[[File:beta3.png|400px|framed|center|Figure 1. This image shows the nuclear process of a beta decay, where a neutron decays to a proton and an electron. The net charge before and after the event is the same by this law.<ref>''Made internally by a member of the Energy Education team''</ref>]] | [[File:beta3.png|400px|framed|center|Figure 1. This image shows the nuclear process of a beta decay, where a neutron decays to a proton and an electron. The net charge before and after the event is the same by this law.<ref>''Made internally by a member of the Energy Education team''</ref>]] | ||
==For Further Reading== | |||
*[[System and surrounding]] | |||
*[[Law of conservation of energy]] | |||
*[[Nuclear decay]] | |||
*[[Mass]] and [[energy]] | |||
*Or explore a [[Special:Random|random page]] | |||
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
[[Category:Uploaded]] | [[Category:Uploaded]] | ||
Revision as of 20:34, 17 July 2018
Law of conservation of charge says that the net charge of an isolated system will always remain constant.[1] This means that any system that is not exchanging mass or energy with its surroundings will never have a different total charge at any two times. For example, if two objects in an isolated system have a net charge of zero, and one object exchanges one million electrons to the other, the object with the excess electrons will be negatively charged and the object with the reduced number of electrons will have a positive charge of the same magnitude. The total charge of the system has not and will never change.[1]
This concept is important for all nuclear reactions—alpha decay, beta decay, gamma decay, etc.— because it allows scientists to predict the composition of the final product in the reaction, shown in Figure 1.[2]
Charged particles are allowed to be created or destroyed, as long as the net charge before and after the creation/destruction stays the same. Therefore this must happen with oppositely charged pairs of matter and anti-matter.[1]
For Further Reading
- System and surrounding
- Law of conservation of energy
- Nuclear decay
- Mass and energy
- Or explore a random page
References
- ↑ 1.0 1.1 1.2 J. Wilson and A. Buffa, "Electric Charge" in College Physics, 5th ed., Upper Saddle River, NJ: Pearson, 2003, ch.15, sec.1, pp.515-516
- ↑ J. Wilson and A. Buffa, "Radioactivity" in College Physics, 5th ed., Upper Saddle River, NJ: Pearson, 2003, ch.29, sec.2, pp.933
- ↑ Made internally by a member of the Energy Education team

